Abstract
Purpose
Racialized economic segregation, a form of structural racism, may drive persistent inequities among patients with breast cancer. We examined whether a composite area-level index of racialized economic segregation was associated with real-world treatment and survival in metastatic breast cancer (mBC).
Methods
We conducted a retrospective cohort study among adult women with mBC using a US nationwide electronic health record-derived de-identified database (2011–2022). Population-weighted quintiles of the index of concentration at the extremes were estimated using census tract data. To identify inequities in time to treatment initiation (TTI) and overall survival (OS), we employed Kaplan–Meier methods and estimated hazard ratios (HR) adjusted for clinical factors.
Results
The cohort included 27,459 patients. Compared with patients from the most privileged areas, those from the least privileged areas were disproportionately Black (36.9% vs. 2.6%) or Latinx (13.2% vs. 2.6%) and increasingly diagnosed with de novo mBC (33.6% vs. 28.9%). Those from the least privileged areas had longer median TTI than those from the most privileged areas (38 vs 31 days) and shorter median OS (29.7 vs 39.2 months). Multivariable-adjusted HR indicated less timely treatment initiation (HR 0.87, 95% CI 0.83, 0.91, p < 0.01) and worse OS (HR 1.19, 95% CI 1.13, 1.25, p < 0.01) among those from the least privileged areas compared to the most privileged areas.
Conclusion
Racialized economic segregation is a social determinant of health associated with treatment and survival inequities in mBC. Public investments directly addressing racialized economic segregation and other forms of structural racism are needed to reduce inequities in cancer care and outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Structural racism is defined as the “differential access to the goods, services, and opportunities of society by race” perpetuating the widespread and unfair treatment of people of color through institutions, practices, and laws [1, 2]. Its impact can be felt in a number of domains, including access to housing, education, and healthcare [3]. A notable manifestation of structural racism is racialized economic segregation—a persistent byproduct of the unfair institutional practice of redlining—which has been associated with inequities in health and healthcare access, including among patients with cancer [4,5,6].
Racialized economic segregation is hypothesized to impact the incidence of cancer and cancer outcomes through its related inequities in access to healthcare facilities, adverse environmental exposures, and the built environment [7]—an association consistently demonstrated across multiple health outcomes with known inequities that exist between Black and White patients [8]. This study offers an in-depth analysis of the implications of racialized economic segregation for patients with metastatic breast cancer (mBC)—a cancer with well-documented racial and ethnic inequities in incidence, treatment, and survival [9, 10].
Building upon related studies that have highlighted the impact of socioeconomic status (SES)—especially lower SES—along with other social determinants of health (SDOH) on health inequities [11,12,13], our research aims to deepen the understanding of how racialized economic segregation contributes to these health inequities. Previous research has shown that measures of residential segregation, redlining and structural racism relate to key outcomes for those with breast cancer, including stage at diagnosis, treatment with surgery, and mortality (both all cause and cancer specific) [4, 5, 14,15,16,17,18]. Collectively, such studies have demonstrated that women with breast cancer who reside in economically marginalized neighborhoods have often experienced notably poorer outcomes, particularly, worse survival. Here, we build on earlier research in several notable ways. First, we examined data from a national cohort, which expands upon previous studies that have examined patient cohorts from individual states, including Maryland, Florida, and New Jersey. Second, we analyzed a contemporary patient cohort that includes those diagnosed with mBC after the onset of the Covid-19 pandemic. Such evidence is needed given other evidence of worsening racial and ethnic inequities during this time period [19]. Third, unique to this study is its examination of treatment initiation, a previously unexplored outcome in the context of racialized economic segregation. This investigation is critical, as timely initiation of treatment is a key factor influencing prognosis and survival [20]. Finally, our study makes a methodological contribution by comparing the sensitivity of our results to the choice and construction of measures of our key exposure. As related studies have examined several different measures of structural racism and racialized economic segregation, it is unclear whether their results are influenced by the specific measure chosen, potentially impacting the comparability of results across studies. Through these contributions, our study addresses several key gaps in the existing literature and underscores the need for policies and interventions that are sensitive to these socioeconomic dimensions, thereby enhancing healthcare outcomes for patients with mBC.
Methods
Data source
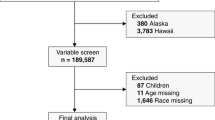
This study used the nationwide Flatiron Health electronic health record (EHR)-derived, de-identified database—a longitudinal database comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction [21, 22]. During the study period, the de-identified data originated from approximately 280 US cancer clinics (approximately 800 sites of care). The study included 27,459 adult women diagnosed with mBC from January 01, 2011 to December 31, 2022 and included women with early-stage breast cancer that later metastasized and de novo mBC [23]. See Fig. 1 for a detailed cohort selection diagram.
Cohort selection diagram. Patients are probabilistically sampled to ensure that an adequate number of patients are queued for chart review during the abstraction phase. A computer-based algorithm for probabilistic selection is used. A small number of patients had an electronic health record of the event of interest prior to the index date and such patients were excluded from those analyses. This is typically a data entry error (e.g., a practice may schedule a treatment that the patient is not able to receive or the date of death is incorrect). mBC metastatic breast cancer
Variables and endpoints
Using data from the American Community Survey (2015–2019, 5-year estimates), we constructed four neighborhood-level measures of racialized economic segregation and SES. Following an approach used by related studies, we categorized each measure into US population-weighted quintiles, with Quintile 1 denoting the least privileged census tracts and Quintile 5 denoting the most privileged census tracts [24, 25]. Our main results focused on the index of concentration at the extremes (ICE), a geospatial measure of racialized economic segregation distinguishing between the least and most privileged groups in an area [23]. By focusing on ICE, the exposure of interest in related research on the implications of racialized economic segregation for health, including mBC outcomes, we aim to contextualize our results within this broader body of literature [4, 5, 17, 18]. Consistent with the terminology used in the original work describing the ICE methodology, we employ the terms “least privileged” versus “most privileged” to distinguish between social groups that have experienced systematic oppression versus systematic advantage from racialized economic segregation.
Thus, our measure of ICE corresponded to the concentration of low-income Black households (least privileged group) and high-income White households (most privileged group) within a census tract following prior studies. Low-income Black households consisted of non-Latinx Black households with incomes below $25,000 per year, and high-income White households consisted of non-Latinx White households with incomes of $100,000 or greater. These income thresholds correspond to the bottom and top quintiles for US household income during the specified years [26]. As a sensitivity analysis, we examined two additional constructions of ICE that compared the concentration of high-income White households to low-income Latinx households and low-income households of Color. Our secondary analysis examined three additional area-level SDOH measures related to racialized economic segregation: (1) The percent of a census tract reporting as non-Latinx Black (Percent Black); (2) The Yost Index, a measure of SES derived from seven inputs reflecting educational attainment, income, housing, and employment within an area [27]; and (3) The Structural Racism Indicator—a newer, composite index of racialized economic segregation based on education, income, household structure, employment, public assistance, occupation, and racial and ethnic composition [28].
Patients included in the cohort were followed from metastatic diagnosis to the first event of interest, death, or last confirmed activity. Time to treatment initiation (TTI) was defined as the start of first-line systemic therapy as evidenced by an EHR-documented order or administration of an antineoplastic therapy determined by oncologist-defined, rule-based lines of therapy. TTI has been recognized as a “patient-centered quality metric” that reflects timely care and has been linked to health outcomes in mBC, including survival [10, 29]. Overall survival (OS) was defined based on documented mortality status. Mortality information was curated from available EHR sources, including structured and unstructured data (e.g., clinician notes). Additionally, the EHR data are linked with commercial obituary data and data from the US Social Security Death Index [30] to supplement dates of death not documented in the EHR. This measure of OS reflects all-cause mortality, as the data sources utilized do not allow for differentiation between cancer-specific and other causes of death.
At the patient level, race and ethnicity values were categorized into mutually exclusive groups: Latinx, non-Latinx Asian (hereafter, Asian), non-Latinx Black (hereafter, Black), non-Latinx White (hereafter White), and Other/Not documented. This latter group included patients without an EHR-documented race or ethnicity and patients with a value of “Other Race.” In the study database, the “Other Race” value is the result of Flatiron Health’s data de-identification process, which masked specific race categories with lower representation in the U.S. population, including American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and patients with multiple races.
Statistical analyses
The Kaplan–Meier product-limit estimator was used to compare TTI and OS between patients residing in areas with differing levels of racialized economic segregation (as measured using ICE quintiles). The Cox model was used to estimate hazard ratios (HR), adjusting for clinical factors, of TTI and OS with a reference group fixed to those residing in the most privileged neighborhoods. HR below one indicated a lower likelihood of initiating treatment (indicative of less timely treatment) and a lower likelihood of death (indicative of increased survival), while HR above one indicated a higher likelihood of initiating treatment and a higher likelihood of death.
The choice of clinical characteristics for adjustment—age at metastatic diagnosis (continuous), year of metastatic diagnosis (categorical), stage at initial diagnosis, molecular subtype, number of metastases, sites of metastasis, and Eastern Cooperative Group (ECOG) performance status—was informed by, and is an extension of, a conceptual framework for health care inequities published in 2003 by the Institute of Medicine (now the National Academy of Medicine) [31]. This framework recognizes that, on average, People of Color have lower socioeconomic profiles than Whites and that such differences in socioeconomic status contribute to inequities in both healthcare access and health outcomes. Thus, according to this framework, socioeconomic status mediates racial/ethnic inequities and, therefore, should not be included as an adjuster in models quantifying the extent of racial/ethnic inequities. Accordingly, we did not adjust for measures of socioeconomic status, like health insurance, as adjustment for socioeconomic status would mitigate the estimated “independent effect” of racialized economic segregation on our outcomes of interest [32, 33].
We also estimated both interactive and stratified models by race and ethnicity using White patients residing in the most privileged areas as the reference group. As an exploratory analysis, we repeated our analysis examining three alternate measures of racialized economic segregation described above. To better characterize the cohort, we also summarized patients’ characteristics by race and ethnicity and examined the distribution of first-line treatments among patients initiating treatment. Statistical analyses were conducted using RStudio Version 2022.12.0 + 353 with R Version 4.2.2 [34, 35]. We tested the proportionality assumption of our Cox proportional hazards models by inspecting Schoenfeld residual plots, employing the cox.zph function within the survival package in R [36].
Results
Characteristics of the overall cohort
Table 1 summarizes the characteristics of the 27,459 patients in the cohort. Median age at metastatic diagnosis was 64 (IQR 54–73). Grouped by stage, 57.3% of patients were diagnosed with early-stage breast cancer that later metastasized, 31.0% presented with de novo breast cancer, and 11.8% had an unknown stage. Among breast cancer subtypes, hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) was the most prevalent, representing 66.1% of cases. This was followed by HR+/HER2+ at 12.3%, HR−/HER2− at 11.6%, and HR−/HER2+ at 4.0%. A small portion, 6.1% of patients, had an unknown subtype. By practice type, 79.9% of patients were associated with community oncology practices, while 20.1% were associated with academic practices. Supplemental Table S1 presents a comparison of the characteristics by race and ethnicity. Compared to White patients, Latinx and Black patients were younger (median age: Latinx = 58, Black = 60, White = 65) more likely to reside in the least privileged areas (ICE Q1: Latinx = 36.2%, Black = 58.4%, White = 7.6%) and differed in insurance coverage (% with Medicaid coverage: Latinx = 5.8%, Black = 4.4%, White = 1.5%). Information on the distribution of first-line treatment among the cohort can be found in Supplemental Table S2. The most common first-line treatment was Aromatase inhibitors (32.1%), followed by chemotherapy (19.6%), Aromatase inhibitors + cyclin-dependent kinase 4/6 inhibitors (17.7%) with the remainder of patients receiving other therapies (30.6%).
Characteristics of the cohort by neighborhood privilege
Table 1 summarizes the characteristics of the cohort by neighborhood privilege. The smallest share of the cohort came from the least privileged neighborhoods (17.2%), and the largest share came from the most privileged neighborhoods (24.0%). Compared to patients from the most privileged areas, those from the least privileged areas exhibited several differences. They were typically younger (median age: 62 vs 64), more likely to be Black (36.9% vs 2.6%) or Latinx individuals (13.2% vs 2.6%) were less often diagnosed with early-stage disease (54.2% vs 58.9%) and more frequently had the aggressive HR−/HER2− subtype (15.6% vs 9.9%).
Median time and adjusted HR of treatment initiation
Figure 2 depicts Kaplan–Meier curves of the risk of initiating first-line treatment within 90 days of metastatic diagnosis by neighborhood privilege. For ease of interpretation, we limited the figure to two curves—comparing the least and most privileged areas—and plotted the inverse risk of initiating first-line treatment (to denote that more patients received treatment as time progressed). At nearly all points in time, patients in the least privileged areas (represented by the orange line) had a lower risk of initiating treatment than patients in the most privileged areas (represented by the blue line).
Kaplan–Meier estimates of treatment initiation comparing patients from the least and most privileged neighborhoods. Treatment initiation denotes first-line treatment initiation within 90 days of metastatic diagnosis. Plot generated in R using the survminer package (version 0.4.9). For ease of interpretation, this figure was limited to two curves—comparing the least and most privileged areas—and plotted the inverse risk of initiating first-line treatment (to denote that more patients received treatment as time progressed). Treatment initiation estimates excluded 3463 patients with a recorded therapy starting prior to metastatic disease that continued beyond 14 days after the index date of metastatic disease
Table 2 presents median TTI by neighborhood privilege. Patients in the least privileged areas had a longer median TTI than patients in the most privileged areas (38 days, 95% CI 36, 40 vs 31 days, 95% CI 29, 32). In addition, those in the least privileged areas had an adjusted HR indicative of less timely treatment initiation (HR 0.905, 95% CI 0.863, 0.950) relative to those in the most privileged areas (reference group).
Median time and adjusted HR of OS
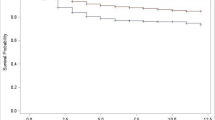
Figure 3 depicts Kaplan–Meier curves of the risk of death within 5 years of metastatic diagnosis by neighborhood privilege. At nearly all points in time, patients in the least privileged areas (represented by the orange line) had a greater risk of death than patients in the most privileged areas (represented by the blue line).
Kaplan–Meier survival estimates comparing patients from the least and most privileged neighborhoods. Kaplan–Meier survival estimates within 5 years of metastatic diagnosis. Survival estimates excluded 181 patients whose recorded death occurred before the index date of metastatic diagnosis. Such exclusion likely results from recording the date of death as year-month for privacy reasons and the use of middle of the month in time-to-event calculations. Plot generated in R using the survminer package (version 0.4.9)
Table 2 presents median OS from metastatic diagnosis by neighborhood privilege. Patients in the least privileged areas had shorter median OS time (29.7 months, 95% CI 28.5, 31.5) than patients in the most privileged areas (39.2 months, 95% CI 37.9, 40.6). In addition, those in the least privileged areas had an adjusted HR indicative of an increased risk of death (HR 1.170, 95% CI 1.107, 1.237) relative to those in the most privileged areas (reference group).
Interactive and stratified results by patient race and ethnicity
Table 3 presents the results from adjusted hazard models incorporating an interaction term between neighborhood privilege and race and ethnicity. Within these models, the reference group denoted White patients from the most privileged neighborhoods. Relative to this group, nearly all other groups had HR indicative of a lower risk of initiating first-line treatment and a greater risk of death. In our analysis of TTI, HR were consistently below one for Black and Asian patients and were similar among those in more and less privileged areas. For Latinx and White patients, HR of TTI were in all but one instance below one, but were often closer to one among those in more privileged areas. In our analysis of OS, HR for Black and White patients were closer to one among those residing in more privileged neighborhoods. Among Asian and Latinx patients, trends across survival HR were less consistent.
Supplementary Table S3 presents Kaplan–Meier estimates of treatment initiation stratified by neighborhood privilege and race and ethnicity and offers additional evidence of racial and ethnic inequities in TTI and rwOS. For example, median TTI was 29 days (95% CI 28, 31) among White patients from the most privileged areas compared to 47 days (95% CI 42, 56) among Latinx patients from the least privileged areas and 38 days (95% CI 35, 40) among Black patients from the least privileged areas. Likewise, for Asian, Black, and White patients, median rwOS was lowest among those from the least privileged areas. Supplementary Table S4 provides evidence of similar inequities in results from adjusted HR of TTI and rwOS stratified by race and ethnicity.
Associations with additional social determinants of health measures
Supplementary Table S5 presents adjusted HR of treatment initiation and OS using alternate constructions of ICE. Compared to our main results, which defined ICE in relation to high-income White and low-income Black households in an area, our results using ICE constructed in relation to low-income Latinx households and low-income households of Color offered similar evidence of inequities. For example, across these three constructions, HR among those from the least privileged areas ranged from 0.872 (95% CI 0.830, 0.915) to 0.905 (95% CI 0.863, 0.950) for TTI and from 1.143 (95% CI 1.081, 1.209) to 1.170 (95% CI 1.107, 1.237) for rwOS.
Table 4 lists correlations between ICE and three additional SDOH measures related to structural racism and SES. These additional measures exhibited strong, positive correlations (> 0.65) with one another, except for Percent Black and the Yost Index (correlation of 0.380). This weaker correlation may reflect the absence of an economic component in the Percent Black measure and the absence of a racial component in the Yost Index.
Table 5 presents the results from an exploratory analysis that utilized these additional measures of structural racism and SES. The top panel presents adjusted HR of the risk of first-line treatment initiation. Among those from neighborhoods with varying concentrations of Black residents, with the exception of Q3, we found no difference in the risk of treatment initiation as all confidence intervals included one. Using the Yost Index, our results were similar to those from our main analysis of ICE, as patients from areas with the lowest SES (Yost Index Quintile 1) had a lower risk of treatment initiation (HR 0.902, 95% CI 0.861, 0.946) than those from the highest SES (Yost Index Quintile 5). Analyses leveraging the Structural Racism Indicator also revealed evidence of inequities in treatment initiation with a lower risk of treatment initiation among those from areas with the highest levels of structural racism (HR 0.954, 95% CI 0.909, 1.002), though the confidence interval included one.
The bottom panel of Table 5 presents adjusted HR of the risk of death. Similar to our main analysis of ICE, our analysis of additional, area-level measures revealed evidence of inequities in OS when comparing the top and bottom quintiles of each measure. Across models, HR among those from areas in the bottom quintile were 1.071 (95% CI 1.012, 1.134) using the Percent Black measure, 1.173 (95% CI 1.112, 1.237) using the Yost Index, and 1.180 (95% CI 1.117, 1.247) using the Structural Racism Indicator.
Discussion
This study offers robust evidence of the association between racialized economic segregation and treatment initiation and survival inequities among patients with mBC. Consistent with prior research [4, 5, 15], patients from less privileged areas were disproportionately Black or Latinx and increasingly diagnosed with de novo mBC. Our analysis, employing a neighborhood-level measure of segregation, showed that patients from less privileged areas faced delayed treatment initiation and shorter survival compared to patients from more privileged neighborhoods, as indicated by both Kaplan–Meier estimates and adjusted HR. Such results are consistent with earlier studies of breast cancer cohorts which examined individual patient data from three states [5, 17, 18] and found that residence in less privileged areas as defined using ICE was associated with an increased risk of death with similar results from an analysis of a national cohort of county-level data [4]. While differences in treatment initiation were more difficult to discern among Asian and Latinx patients due to wider confidence intervals (i.e., smaller sample sizes), sharp inequities were evident among Black patients, especially those from the least privileged areas. Notably, Black patients from even the most privileged areas experienced shorter median survival times compared to White patients living in the least privileged neighborhoods, underscoring the deep impact of racialized economic segregation on OS among patients with mBC. Further evidence of these findings comes from our exploratory analyses using alternate SDOH measures related to structural racism, which showed similar inequities. These results collectively underscore the critical influence that racialized economic segregation plays in shaping health outcomes for patients with mBC.
The historical consequences of racialized economic segregation have been long-lasting and profound through its contribution to limited upward mobility and the wealth gap between different racial and ethnic groups [6, 37]. Our study substantially expands the understanding of its consequences on health from past studies in several ways. First, we examined how racialized economic segregation related to both treatment initiation and survival, building on past studies which solely focused on investigating inequities in survival [4, 5]. Second, we utilized a tract-level measure of racialized economic segregation, differing from a prior study that examined a less granular county-level measure of segregation [4]. An advantage of tract-level data is its potential to offer a more nuanced reflection of racialized economic segregation, given that census tracts are small, relatively permanent statistical subdivisions of a county with a lower median population size accounting for within-county segregation [38]. Third, we examined multiple alternative measures of segregation in our sensitivity analysis, providing a significant methodological contribution. Findings from that analysis suggest that while multiple approaches can be used to measure the implications of racialized economic segregation among patients with mBC, the choice of these approaches should be aligned with a conceptual framework that takes into account the relevant associations of interest. Finally, we examined data from a national and contemporary cohort of patients from across the USA, building on some of the past studies that relied on data from a single state during the pre-pandemic period [17].
There are several ways in which structural racism influences cancer care and cancer outcomes in the USA, and understanding such pathways is essential for designing effective solutions [11,12,13, 39,40,41]. Environmental injustices like the disproportionate placement of power plants near communities of color amplify cancer risks for those communities [42]. The structure of the US healthcare system acts as a barrier to preventive cancer screenings, with Latinx and Black adults being three times less likely to have insurance compared to White adults [43]. Policies that divert healthcare resources away from predominantly Black neighborhoods, like closures of publicly funded hospitals, also contribute to this inequity [44]. With respect to our findings, racialized economic segregation may influence health preferences and behaviors, inequities in the healthcare marketplace within communities, and discrepancies in environmental risk factors affecting health needs. A considerable proportion of the observed racial and ethnic inequities in breast cancer are attributed to geographic-level factors that are linked to the racial and ethnic composition of neighborhoods and communities [45]. Additionally, racial and ethnic inequities in the stage at which breast cancer is diagnosed likely reflects differential access to early screening and preventive measures, highlighting the urgent need for policies promoting equitable healthcare access. Ultimately, reducing racial and ethnic inequities in cancer care and cancer outcomes requires public investments in areas that directly address structural racism (e.g., enhancing funding for medical facilities in segregated neighborhoods, implementing policies for equitable healthcare reimbursements across different communities, and establishing community health outreach programs to improve access).
Limitations
This study has its limitations. As with any observational study, there is potential for unmeasured confounders to influence our survival estimates as notably, our dataset lacked comprehensive information on comorbidities. Additionally, our analysis was limited to a measure of all-cause mortality, precluding a more precise examination of cancer-related mortality, although a related study examining both all-cause and cancer-specific mortality found similar results across outcomes [15]. The residential location, census tract, for patients was geocoded to the most recent address documented in the EHR, and measures of racialized economic segregation may not fully capture this experience at diagnosis, throughout a patient’s cancer journey, or before diagnosis. Our analysis was also focused on female patients with mBC, given the limited number of male patients in our dataset, which introduces uncertainty about the generalizability of our results to all patients with mBC, including male patients and those diagnosed with early-stage disease. Finally, the generalizability of our results to other cancers and diseases also remains uncertain. While our results suggest that multiple measures of neighborhood structural racism can be used to detect inequities among patients with mBC, further research is needed to establish the interchangeability of these measures in research on other disease states. Nevertheless, concerns about the generalizability of our results across the USA are mitigated by a prior analysis comparing the study dataset to two other national cohorts, SEER and NPCR, which found these mBC cohorts had similar clinical and demographic characteristics [21].
Conclusion
Utilizing the index of concentration at the extremes, a measure of racialized economic segregation, we found that patients residing in the least privileged areas experienced a longer median time before initiating first-line therapy for mBC and a shorter median survival than patients in the most privileged areas. These findings were consistent after adjusting for demographic and clinical factors. Notably, we observed that Black patients in less privileged areas were more likely to experience worse outcomes compared to White patients in the most privileged ones. Our study underscores the importance of adopting policies that specifically address structural racism, as they hold the potential to enhance outcomes for patients with mBC.
Data availability
The data that support the findings of this study were originated by and are the property of Flatiron Health, Inc., which has restrictions prohibiting the authors from making the data set publicly available. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to publicationdataaccess@flatiron.com.
References
Jones CP (2000) Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health 90:1212–1215. https://doi.org/10.2105/ajph.90.8.1212
Braveman PA, Arkin E, Proctor D et al (2022) Systemic and structural racism: definitions, examples, health damages, and approaches to dismantling. Health Aff (Millwood) 41:171–178. https://doi.org/10.1377/hlthaff.2021.01394
Bailey ZD, Feldman JM, Bassett MT (2020) How structural racism works—racist policies as a root cause of U.S. racial health inequities. N Engl J Med 384:768–773. https://doi.org/10.1056/nejmms2025396
Zhang L, Gong R, Shi L et al (2023) Association of residential racial and economic segregation with cancer mortality in the US. JAMA Oncol 9:122–126. https://doi.org/10.1001/jamaoncol.2022.5382
Wiese D, Stroup AM, Crosbie A et al (2019) The impact of neighborhood economic and racial inequalities on the spatial variation of breast cancer survival in New Jersey. Cancer Epidemiology Prev Biomark 28:1958–1967. https://doi.org/10.1158/1055-9965.epi-19-0416
Aaronson D, Hartley D, Mazumder B (2021) The effects of the 1930s HOLC “Redlining” maps. Am Econ J Econ Pol 13:355–392. https://doi.org/10.1257/pol.20190414
Gaskin DJ, Dinwiddie GY, Chan KS, McCleary R (2011) Residential segregation and disparities in health care services utilization. Méd Care Res Rev 69:158–175. https://doi.org/10.1177/1077558711420263
Landrine H, Corral I (2009) Separate and unequal: residential segregation and black health disparities. Ethn Dis 19:179–184
Surveillance Research Program, National Cancer Institute (2023) SEER*Explorer: an interactive website for SEER cancer statistics. https://seer.cancer.gov/statistics-network/explorer/. Accessed 16 Nov 2023
Whitaker KD, Wang X, Ascha M et al (2022) Racial inequities in second-line treatment and overall survival among patients metastatic breast cancer. Breast Cancer Res Treat 196:163–173. https://doi.org/10.1007/s10549-022-06701-5
Huang H-C, Smart MH, Zolekar A et al (2022) Impact of socioeconomic status and rurality on cancer-specific survival among women with de novo metastatic breast cancer by race/ethnicity. Breast Cancer Res Treat 193:707–716. https://doi.org/10.1007/s10549-022-06603-6
Shariff-Marco S, Yang J, John EM et al (2014) Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the neighborhood and breast cancer study. Cancer Epidemiol Prev Biomark 23:793–811. https://doi.org/10.1158/1055-9965.epi-13-0924
Coughlin SS (2019) Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 177:537–548. https://doi.org/10.1007/s10549-019-05340-7
Poulson MR, Beaulieu-Jones BR, Kenzik KM et al (2020) Residential racial segregation and disparities in breast cancer presentation, treatment, and survival. Ann Surg 273:3–9. https://doi.org/10.1097/sla.0000000000004451
Beyer KMM, Zhou Y, Laud PW et al (2021) Mortgage lending bias and breast cancer survival among older women in the United States. J Clin Oncol 39:2749–2757. https://doi.org/10.1200/jco.21.00112
Plascak JJ, Beyer K, Xu X et al (2022) Association between residence in historically redlined districts indicative of structural racism and racial and ethnic disparities in breast cancer outcomes. JAMA Netw Open 5:e2220908. https://doi.org/10.1001/jamanetworkopen.2022.20908
Goel N, Westrick AC, Bailey ZD et al (2022) Structural racism and breast cancer-specific survival. Ann Surg 275:776–783. https://doi.org/10.1097/sla.0000000000005375
Connor AE, Kaur M, Dibble KE et al (2021) Racialized economic segregation and breast cancer mortality among women in Maryland. Cancer Epidemiol Prev Biomark. https://doi.org/10.1158/1055-9965.epi-21-0923
Centers for Disease Control and Prevention (2023) Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Accessed 8 Mar 2024
Waks AG, King TA, Winer EP (2015) Timeliness in breast cancer treatment—the sooner, the better. JAMA Oncol 2:1–3. https://doi.org/10.1001/jamaoncol.2015.4506
Ma X, Long L, Moon S et al (2020) Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. https://doi.org/10.1101/2020.03.16.20037143
Birnbaum B, Nussbaum N, Seidl-Rathkopf K et al (2020) Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv Preprint. https://doi.org/10.48550/arxiv.2001.09765
Krieger N, Waterman PD, Spasojevic J et al (2016) Public health monitoring of privilege and deprivation with the index of concentration at the extremes. Am J Public Health 106:256–263. https://doi.org/10.2105/ajph.2015.302955
Chambers BD, Baer RJ, McLemore MR, Jelliffe-Pawlowski LL (2019) Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality—California, 2011–2012. J Urban Health 96:159–170. https://doi.org/10.1007/s11524-018-0272-4
Sonderlund AL, Charifson M, Schoenthaler A et al (2022) Racialized economic segregation and health outcomes: a systematic review of studies that use the Index of concentration at the extremes for race, income, and their interaction. PLoS ONE 17:e0262962. https://doi.org/10.1371/journal.pone.0262962
Krieger N, Chen JT, Waterman PD (2020) The public health disparities geocoding project: COVID-19 resources. In: Using the methods of the public health disparities geocoding project to monitor COVID-19 inequities and guide action for health justice. https://www.hsph.harvard.edu/thegeocodingproject/covid-19-resources/. Accessed 10 Jul 2023
Yost K, Perkins C, Cohen R et al (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711. https://doi.org/10.1023/a:1011240019516
Abraham IE, Rauscher GH, Patel AA et al (2022) Structural racism is a mediator of disparities in acute myeloid leukemia outcomes. Blood 139:2212–2226. https://doi.org/10.1182/blood.2021012830
Parikh RB, Takvorian SU, Vader D et al (2022) Impact of the COVID-19 pandemic on treatment patterns for patients with solid cancer in the United States. J Natl Cancer Inst 114:571–578. https://doi.org/10.1093/jnci/djab225
Curtis MD, Griffith SD, Tucker M et al (2018) Development and validation of a high-quality composite real-world mortality endpoint. Heal Serv Res 53:4460–4476. https://doi.org/10.1111/1475-6773.12872
Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care (2003) Unequal treatment: confronting racial and ethnic disparities in health care. National Academies Press, Washington
Samuel CA, Landrum MB, McNeil BJ et al (2014) Racial disparities in cancer care in the veterans affairs health care system and the role of site of care. Am J Public Health 104:S562–S571. https://doi.org/10.2105/ajph.2014.302079
McGuire TG, Alegria M, Cook BL et al (2006) Implementing the institute of medicine definition of disparities: an application to mental health care. Health Serv Res 41:1979–2005. https://doi.org/10.1111/j.1475-6773.2006.00583.x
Posit Team (2022) RStudio: integrated development environment for R. Posit Software, PBC, Boston
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Therneau TM (2024) A package for survival analysis in R. R Package Version
Chetty R, Hendren N, Katz LF (2016) The effects of exposure to better neighborhoods on children: new evidence from the moving to opportunity experiment. Am Econ Rev 106:855–902. https://doi.org/10.1257/aer.20150572
Vo A, Tao Y, Li Y, Albarrak A (2023) The association between social determinants of health and population health outcomes: ecological analysis. JMIR Public Heal Surveill 9:e44070. https://doi.org/10.2196/44070
Vrdoljak E, Gligorov J, Wierinck L et al (2021) Addressing disparities and challenges in underserved patient populations with metastatic breast cancer in Europe. Breast 55:79–90. https://doi.org/10.1016/j.breast.2020.12.005
MacKinnon JA, Duncan RC, Huang Y et al (2007) Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiol Biomark Prev 16:756–762. https://doi.org/10.1158/1055-9965.epi-06-0392
Haas JS, Earle CC, Orav JE et al (2008) Racial segregation and disparities in breast cancer care and mortality. Cancer 113:2166–2172. https://doi.org/10.1002/cncr.23828
Tessum CW, Paolella DA, Chambliss SE et al (2021) PM2.5 polluters disproportionately and systemically affect people of color in the United States. Sci Adv. https://doi.org/10.1126/sciadv.abf4491
Branch B, Conway D (2022) Health Insurance coverage by race and Hispanic origin: 2021. United States Census Bureau, Washington
Eberth JM, Hung P, Benavidez GA et al (2022) The problem of the color line: spatial access to hospital services for minoritized racial and ethnic groups. Heal Aff 41:237–246. https://doi.org/10.1377/hlthaff.2021.01409
Hoskins KF, Calip GS, Huang H-C et al (2023) Association of social determinants and tumor biology with racial disparity in survival from early-stage, hormone-dependent breast cancer. JAMA Oncol 9:536–545. https://doi.org/10.1001/jamaoncol.2022.7705
Acknowledgements
The authors thank Darren Johnson of Flatiron Health, Inc., New York, USA for providing editorial support, which was funded by Flatiron Health, Inc., New York, USA in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
This study was sponsored by Flatiron Health, Inc., which is an independent member of the Roche group.
Author information
Authors and Affiliations
Contributions
HP, GSC, and JSG contributed to conceptualization and design of the study. HP, GSC, and JSG established the methodology. Validation was conducted by JSG. Data analysis was performed by HP. The first draft of the manuscript was written by HP and GSC. All authors contributed to manuscript review and editing. Supervision was provided by CAR. GSC provided project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
At the time of this study, HP, GSC, AP, CAR, and JSG report employment at Flatiron Health, Inc., which is an independent member of the Roche Group and stock ownership in Roche. GSC reports current employment at AbbVie Inc.
Ethical approval and consent to participate
The IRB of WCG IRB gave ethical approval for the study protocol prior to study conduct and included a waiver of informed consent (Approval ID: 420180044).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pittell, H., Calip, G.S., Pierre, A. et al. Racialized economic segregation and inequities in treatment initiation and survival among patients with metastatic breast cancer. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07319-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07319-5