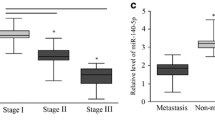
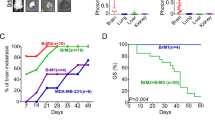
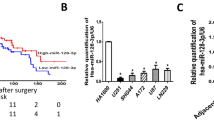
Abstract
Purpose
Extravasation of triple-negative (TN) metastatic breast cancer (BC) cells through the brain endothelium (BE) is a critical step in brain metastasis (BM). During extravasation, metastatic cells induce alteration in the inter-endothelial junctions and transmigrate through the endothelial barrier. Transmigration of metastatic cells is mediated by the upregulation of cyclooxygenase-2 (COX-2) that induces matrix metalloproteinase-1 (MMP-1) capable of degrading inter-endothelial junctional proteins. Despite their important role in BM, the molecular mechanisms upregulating COX-2 and MMP-1 in TNBC cells remain poorly understood. In this study, we unraveled a synergistic effect of a pair of micro-RNAs (miR-26b-5p and miR-101-3p) on COX-2 expression and the brain transmigration ability of BC cells.
Methods
Using a gain-and-loss of function approach, we modulated levels of miR-26b-5p and miR-101-3p in two TNBC cell lines (the parental MDA-MB-231 and its brain metastatic variant MDA-MB-231-BrM2), and examined the resultant effect on COX-2/MMP-1 expression and the transmigration of cancer cells through the BE.
Results
We observed that the dual inhibition of miR-26b-5p and miR-101-3p in BC cells results in higher increase of COX-2/MMP-1 expression and a higher trans-endothelial migration compared to either micro-RNA alone. The dual restoration of both micro-RNAs exerted a synergistic inhibition on COX-2/MMP-1 by targeting COX-2 and potentiated the suppression of trans-endothelial migration compared to single micro-RNA.
Conclusion
These findings provide new insights on a synergism between miR-26-5p and miR-101-3p in regulating COX-2 in metastatic TNBC cells and shed light on miR-26-5p and miR-101-3p as prognostic and therapeutic targets that can be exploited to predict or prevent BM.








Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary material.
References
Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK (2015) Emerging strategies for treating brain metastases from breast cancer. Cancer Cell 27:163–175. https://doi.org/10.1016/j.ccell.2015.01.001
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28:3271–3277. https://doi.org/10.1200/JCO.2009.25.9820
Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S et al (2014) Metastatic breast cancer subtypes and central nervous system metastases. Breast 23:623–628. https://doi.org/10.1016/j.breast.2014.06.009
Uhm JE, Park YH, Yi SY, Cho EY, Choi YL, Lee SJ et al (2009) Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer 124:1457–1462. https://doi.org/10.1002/ijc.24090
Custódio-Santos T, Videira M, Brito MA (2017) Brain metastasization of breast cancer. Biochim Biophys Acta 1868:132–147. https://doi.org/10.1016/j.bbcan.2017.03.004
Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Bauer HS, Krizbai IA, Bauer H, Traweger A (2014) “You Shall Not Pass”—tight junctions of the blood brain barrier. Front Neurosci 8:392. https://doi.org/10.3389/fnins.2014.00392
Rodriguez PL, Jiang S, Fu Y, Avraham S, Avraham HK (2014) The proinflammatory peptide substance P promotes blood–brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int J Cancer 134:1034–1044. https://doi.org/10.1002/ijc.28433
Avraham HK, Jiang S, Fu Y, Nakshatri H, Ovadia H, Avraham S (2014) Angiopoietin-2 mediates blood–brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol 232:369–381. https://doi.org/10.1002/path.4304
Lee TH, Avraham HK, Jiang S, Avraham S (2003) Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278:5277–5284. https://doi.org/10.1074/jbc.M210063200
Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y et al (2015) Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem 290:9842–9854. https://doi.org/10.1074/jbc.M114.602185
Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX et al (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459:1005–1009. https://doi.org/10.1038/nature08021
Liu H, Kato Y, Erzinger SA, Kiriakova GM, Qian Y, Palmieri D et al (2012) The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer 12:583. https://doi.org/10.1186/1471-2407-12-583
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531. https://doi.org/10.1038/nrg1379
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16:203–222. https://doi.org/10.1038/nrd.2016.246
Oliveto S, Mancino M, Manfrini N, Biffo S (2017) Role of microRNAs in translation regulation and cancer. World J Biol Chem 8:45. https://doi.org/10.4331/wjbc.v8.i1.45
Kulyte A, Belarbi Y, Lorente-Cebrian S, Bambace C, Arner E, Daub CO et al (2014) Additive effects of microRNAs and transcription factors on CCL2 production in human white adipose tissue. Diabetes 63:1248–1258. https://doi.org/10.2337/db13-0702
Xu Y, Zhu W, Wang Z, Yuan W, Sun Y, Liu H et al (2015) Combinatorial microRNAs suppress hypoxia-induced cardiomyocytes apoptosis. Cell Physiol Biochem 37:921–932. https://doi.org/10.1159/000430219
Zhang J, Pham VVH, Liu L, Xu T, Truong B, Li J et al (2019) Identifying miRNA synergism using multiple-intervention causal inference. BMC Bioinform 20:613. https://doi.org/10.1186/s12859-019-3215-5
Vandenwijngaert S, Ledsky CD, Agha O, Wu C, Hu D, Bagchi A et al (2018) MicroRNA-425 and microRNA-155 cooperatively regulate atrial natriuretic peptide expression and cGMP production. PLoS ONE 13:e0196697. https://doi.org/10.1371/journal.pone.0196697
Chen X, Zhao W, Yuan Y, Bai Y, Sun Y, Zhu W et al (2017) MicroRNAs tend to synergistically control expression of genes encoding extensively-expressed proteins in humans. PeerJ 5:e3682. https://doi.org/10.7717/peerj.3682
Zhu W, Zhao Y, Xu Y, Sun Y, Wang Z, Yuan W et al (2013) Dissection of protein interactomics highlights microRNA synergy. PLoS ONE 8:e63342. https://doi.org/10.1371/journal.pone.0063342
Liu Y, Li H, Zhao C, Jia H (2018) MicroRNA-101 inhibits angiogenesis via COX-2 in endometrial carcinoma. Mol Cell Biochem 448:61–69. https://doi.org/10.1007/s11010-018-3313-0
Cao J, Guo T, Dong Q, Zhang J, Li Y (2015) miR-26b is downregulated in human tongue squamous cell carcinoma and regulates cell proliferation and metastasis through a COX-2-dependent mechanism. Oncol Rep 33:974–980. https://doi.org/10.3892/or.2014.3648
Strillacci A, Griffoni C, Sansone P, Paterini P, Piazzi G, Lazzarini G et al (2009) MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp Cell Res 315:1439–1447. https://doi.org/10.1016/j.yexcr.2008.12.010
Li J, Kong X, Zhang J, Luo Q, Li X, Fang L (2013) MiRNA-26b inhibits proliferation by targeting PTGS2 in breast cancer. Cancer Cell Int 13:7. https://doi.org/10.1186/1475-2867-13-7
Xia M, Duan ML, Tong JH, Xu JG (2015) MiR-26b suppresses tumor cell proliferation, migration and invasion by directly targeting COX-2 in lung cancer. Eur Rev Med Pharmacol Sci 19(24):4728–4737
Harati R, Mohammad MG, Tlili A, El-Awady RA, Hamoudi R (2020) Loss of miR-101-3p promotes transmigration of metastatic breast cancer cells through the brain endothelium by inducing COX-2/MMP1 signaling. Pharmaceuticals 13:144. https://doi.org/10.3390/ph13070144
Xing F, Sharma S, Liu Y, Mo YY, Wu K, Zhang YY et al (2015) miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene 34:4890–4900. https://doi.org/10.1038/onc.2014.412
Weksler B, Romero IA, Couraud PO (2013) The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10:16. https://doi.org/10.1186/2045-8118-10-16
Poller B, Gutmann H, Krähenbühl S, Weksler B, Romero I, Couraud P-O et al (2008) The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem 107:1358–1368. https://doi.org/10.1111/j.1471-4159.2008.05730.x
Harati R, Benech H, Villégier AS, Mabondzo A (2013) P-Glycoprotein, breast cancer resistance protein, organic anion transporter 3, and transporting peptide 1a4 during blood-brain barrier maturation: involvement of wnt/β-catenin and endothelin-1 signaling. Mol Pharm 10:1566–1580. https://doi.org/10.1021/mp300334r
Harati R, Villégier A-S, Banks WA, Mabondzo A (2012) Susceptibility of juvenile and adult blood–brain barrier to endothelin-1: regulation of P-glycoprotein and breast cancer resistance protein expression and transport activity. J Neuroinflammation 9:765. https://doi.org/10.1186/1742-2094-9-273
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Harati R, Hafezi S, Mabondzo A, Tlili A (2020) Silencing miR-202-3p increases MMP-1 and promotes a brain invasive phenotype in metastatic breast cancer cells. PLoS ONE 15:e0239292–e0239292. https://doi.org/10.1371/journal.pone.0239292
Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P, Luissint AC, di Tommaso A, Deshayes F et al (2012) Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS ONE 7:e35667. https://doi.org/10.1371/journal.pone.0035667
Fu J, Zhang N, Chou JH, Dong HJ, Lin SF, Ulrich-Merzenich GS et al (2016) Drug combination in vivo using combination index method: taxotere and T607 against colon carcinoma HCT-116 xenograft tumor in nude mice. Synergy 3:15–30. https://doi.org/10.1016/j.synres.2016.06.001
Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621. https://doi.org/10.1124/pr.58.3.10
Chou TC (2018) The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy 7:49–50. https://doi.org/10.1016/j.synres.2018.04.001
Yadav SS, Li J, Stockert JA, O’Connor J, Herzog B, Elaiho C et al (2016) Combination effect of therapies targeting the PI3K- and AR-signaling pathways in prostate cancer. Oncotarget 7:76181–76196. https://doi.org/10.18632/oncotarget.12771
Lai X, Eberhardt M, Schmitz U, Vera J (2019) Systems biology-based investigation of cooperating microRNAs as monotherapy or adjuvant therapy in cancer. Nucleic Acids Res 47:7753–7766. https://doi.org/10.1093/nar/gkz638
Jin HY, Gonzalez-Martin A, Miletic AV, Lai M, Knight S, Sabouri-Ghomi M et al (2015) Transfection of microRNA mimics should be used with caution. Front Genet. https://doi.org/10.3389/fgene.2015.00340
Mazhar D, Ang R, Waxman J (2006) COX inhibitors and breast cancer. Br J Cancer 94:346–350. https://doi.org/10.1038/sj.bjc.6602942
Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K (2019) Cyclooxygenase-2 in cancer: a review. J Cell Physiol 234:5683–5699. https://doi.org/10.1002/jcp.27411
Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ (2004) COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer 90:423–429. https://doi.org/10.1038/sj.bjc.6601534
Timoshenko AV, Xu G, Chakrabarti S, Lala PK, Chakraborty C (2003) Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp Cell Res 289:265–274. https://doi.org/10.1016/S0014-4827(03)00269-6
Majumder M, Xin X, Liu L, Girish GV, Lala PK (2014) Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci 105:1142–1151. https://doi.org/10.1111/cas.12475
Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC et al (2004) Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci 101(2):591–596. https://doi.org/10.1073/pnas.2535911100
Li J, Hao Q, Cao W, Vadgama JV, Wu Y (2018) Celecoxib in breast cancer prevention and therapy. Cancer Manag Res 10:4653–4667. https://doi.org/10.2147/CMAR.S178567
Denkert C, Winzer KJ, Müller BM, Weichert W, Pest S, Köbel M et al (2003) Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 97:2978–2987. https://doi.org/10.1002/cncr.11437
Shim JY, An HJ, Lee YH, Kim SK, Lee KP, Lee KS (2003) Overexpression of cyclooxygenase-2 Is associated with breast carcinoma and its poor prognostic factors. Mod Pathol 16:1199–1204. https://doi.org/10.1097/01.MP.0000097372.73582.CB
Tan KB, Yong WP, Putti TC (2004) Cyclooxygenase-2 expression: a potential prognostic and predictive marker for high-grade ductal carcinoma in situ of the breast. Histopathology 44:24–28. https://doi.org/10.1111/j.1365-2559.2004.01774.x
Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A et al (2005) COX-2 expression in DCIS: correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology 46:561–568. https://doi.org/10.1111/j.1365-2559.2005.02132.x
Harris RE (2014) Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J Clin Oncol 5:677. https://doi.org/10.5306/wjco.v5.i4.677
Zhang Z, Chen F, Shang L (2018) Advances in antitumor effects of NSAIDs. Cancer Manag Res 10:4631–4640. https://doi.org/10.2147/CMAR.S175212
Winer A, Adams S, Mignatti P (2018) Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther 17:1147–1155. https://doi.org/10.1158/1535-7163.MCT-17-0646
Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM et al (2007) Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res 67:4190–4198. https://doi.org/10.1158/0008-5472.CAN-06-3316
He Y, Liu H, Jiang L, Rui B, Mei J, Xiao H (2019) miR-26 induces apoptosis and inhibits autophagy in non-small cell lung cancer cells by suppressing TGF-β1-JNK signaling pathway. Front Pharmacol 9:1509. https://doi.org/10.3389/fphar.2018.01509
Wang XJ, Yan ZJ, Luo GC, Chen YY, Bai PM (2020) miR-26 suppresses renal cell cancer via down-regulating coronin-3. Mol Cell Biochem 463:137–146. https://doi.org/10.1007/s11010-019-03636-2
Li J, Liang Y, Lv H, Meng H, Xiong G, Guan X et al (2017) miR-26a and miR-26b inhibit esophageal squamous cancer cell proliferation through suppression of c-MYC pathway. Gene 625:1–9. https://doi.org/10.1016/j.gene.2017.05.001
Zhu L, Chen Y, Nie K, Xiao Y, Yu H (2018) MiR-101 inhibits cell proliferation and invasion of pancreatic cancer through targeting STMN1. Cancer Biomark 23:301–309. https://doi.org/10.3233/CBM-181675
Liu P, Ye F, Xie X, Li X, Tang H, Li S et al (2016) mir-101–3p is a key regulator of tumor metabolism in triple negative breast cancer targeting AMPK. Oncotarget 7(23):35188–35198. https://doi.org/10.18632/oncotarget.9072
Liu X, Tang H, Chen J, Song C, Yang L, Liu P et al (2015) MicroRNA-101 inhibits cell progression and increases paclitaxel sensitivity by suppressing MCL-1 expression in human triple-negative breast cancer. Oncotarget 6:20070–20083. https://doi.org/10.18632/oncotarget.4039
Zhang X, Gao D, Fang K, Guo Z, Li L (2019) Med19 is targeted by miR-101–3p/miR-422a and promotes breast cancer progression by regulating the EGFR/MEK/ERK signaling pathway. Cancer Lett 444:105–115. https://doi.org/10.1016/j.canlet.2018.12.008
Li L, Shao MY, Zou SC, Xiao ZF, Chen ZC (2019) MiR-101-3p inhibits EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM44. J Neurooncol 141:19–30. https://doi.org/10.1007/s11060-018-2973-7
Han L, Chen W, Xia Y, Song Y, Zhao Z, Cheng H et al (2018) MiR-101 inhibits the proliferation and metastasis of lung cancer by targeting zinc finger E-box binding homeobox1. Am J Transl Res 10(4):1172–1183
Yan S, Shan X, Chen K, Liu Y, Yu G, Chen Q et al (2018) LINC00052/miR-101-3p axis inhibits cell proliferation and metastasis by targeting SOX9 in hepatocellular carcinoma. Gene 679:138–149. https://doi.org/10.1016/j.gene.2018.08.038
Bao RF, Shu YJ, Hu YP, Wang XA, Zhang F, Liang HB et al (2016) miR-101 targeting ZFX suppresses tumor proliferation and metastasis by regulating the MAPK/Erk and Smad pathways in gallbladder carcinoma. Oncotarget 7:22339–22354. https://doi.org/10.18632/oncotarget.7970
Ding Q, Wang Y, Zuo Z, Gong Y, Krishnamurthy S, Li CW et al (2018) Decreased expression of microRNA-26b in locally advanced and inflammatory breast cancer. Hum Pathol 77:121–129. https://doi.org/10.1016/j.humpath.2018.04.002
Funding
This work was supported by the Terry Fox Foundation’s International Run Program (Grant ref. I1032). R Hamoudi is funded by Al-Jalila Foundation (Grant No: AJF201741). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
RH designed the study, performed research, analyzed, and interpreted the data and wrote the manuscript; AT, MM, and GK contributed to the luciferase reporter assay; AT, AM, GK, and RH provided support with analysis of data and interpretation of results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest/competing interests.
Ethical approval
All experiments were approved by the University of Sharjah animal care and use committee and conducted according to the UOS directives for animal care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harati, R., Mabondzo, A., Tlili, A. et al. Combinatorial targeting of microRNA-26b and microRNA-101 exerts a synergistic inhibition on cyclooxygenase-2 in brain metastatic triple-negative breast cancer cells. Breast Cancer Res Treat 187, 695–713 (2021). https://doi.org/10.1007/s10549-021-06255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06255-y