Abstract
Patients with breast cancer experience treatment-related symptoms which are unlike side effects associated with therapy such as surgery, chemotherapy or radiation. These symptoms are collectively referred to as symptoms cluster and include concurrent physical and/or psychosocial symptoms. Psychoneurological symptom cluster has been used to describe fatigue, mood changes, cognitive and sleep disturbances and pain seen in patients diagnosed with cancer. The etiology of psychoneurological symptom cluster is unclear; however, inflammation has been shown to play a role. High quality diets defined as diets rich in fruits, vegetables, whole grains and polyunsaturated fatty acids and low in added sugar have been shown to decrease inflammation in patients. This article reviews the role of inflammation and high quality diet on the prevalence of psychoneurological symptoms clusters.


Similar content being viewed by others
References
Dodd MJ, Miaskowski C, Paul SM (2001) Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 28(3):465–470
Chen ML, Tseng HC (2006) Symptom clusters in cancer patients. Support Care Cancer 14(8):825–830
Barsevick A (2016) Defining the symptom cluster: how far have we come? Semin Oncol Nurs 32(4):334–350
So WK et al (2009) The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum 36(4):E205–E214
Albusoul RM et al (2017) Symptom clusters change over time in women receiving adjuvant chemotherapy for breast cancer. J Pain Symptom Manag 53(5):880–886
Kim HJ et al (2008) Treatment-related symptom clusters in breast cancer: a secondary analysis. J Pain Symptom Manag 36(5):468–479
Barsevick AM et al (2006) Symptom cluster research: conceptual, design, measurement and analysis issues. J Pain Symptom Manag 31(1):85–95
Dodd MJ et al (2010) The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs 14(2):101–110
Kim HJ, McDermott PA, Barsevick AM (2014) Comparison of groups with different patterns of symptom cluster intensity across the breast cancer treatment trajectory. Cancer Nurs 37(2):88–96
Hamash KI et al (2018) The effect of the pain symptom cluster on performance in women diagnosed with advanced breast cancer: the mediating role of the psychoneurological symptom cluster. Pain Manag Nurs. https://doi.org/10.1016/j.pmn.2018.05.002
Janz NK et al (2007) Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 16(9):1348–1361
Marshall SA et al (2016) Symptom clusters in women with breast cancer: an analysis of data from social media and a research study. Qual Life Res 25(3):547–557
Sarenmalm EK, Browall M, Gaston-Johansson F (2014) Symptom burden clusters: a challenge for targeted symptom management. A longitudinal study examining symptom burden clusters in breast cancer. J Pain Symptom Manag 47(4):731–741
Glaus A et al (2006) Fatigue and menopausal symptoms in women with breast cancer undergoing hormonal cancer treatment. Ann Oncol 17(5):801–806
Seib C et al (2017) Menopausal symptom clusters and their correlates in women with and without a history of breast cancer: a pooled data analysis from the Women’s Wellness Research Program. Menopause 24(6):624–634
Ridner SH (2005) Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 13(11):904–911
Ridner SH et al (2018) Development and validation of the lymphedema symptom intensity and distress survey-lower limb. Lymphat Res Biol 16(6):538–546
Ahmed RL et al (2008) Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol 26(35):5689–5696
Kirkova J et al (2011) Cancer symptom clusters: clinical and research methodology. J Palliat Med 14(10):1149–1166
Kissane DW et al (2004) Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Aust N Z J Psychiatry 38(5):320–326
Davis LE et al (2018) Patient-reported symptoms after breast cancer diagnosis and treatment: a retrospective cohort study. Eur J Cancer 101:1–11
Groenvold M et al (2007) Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 105(2):209–219
Chen SJ et al (2016) Association between depressive disorders and risk of breast cancer recurrence after curative surgery. Medicine 95(33):e4547
Kanani R et al (2016) The association of mood disorders with breast cancer survival: an investigation of linked cancer registration and hospital admission data for South East England. Psycho-Oncology 25(1):19–27
De Aguiar SS, Bergmann A, Mattos IE (2014) Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res 23(2):627–637
Thornton LM, Andersen BL, Blakely WP (2010) The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol 29(3):333–337
Kim HJ et al (2012) Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs 35(6):E1–E20
Jaremka LM et al (2013) Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology 38(8):1310–1317
Dantzer R (2004) Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500(1–3):399–411
Seruga B et al (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8(11):887–899
Bower JE, Ganz PA, Aziz N (2005) Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 67(2):277–280
Schiepers OJ, Wichers MC, Maes M (2005) Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 29(2):201–217
Erez N et al (2013) Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun 437(3):397–402
Erez N et al (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147
Fernandez-Garcia B et al (2016) Prognostic significance of inflammatory factors expression by stroma from breast carcinomas. Carcinogenesis 37(8):768–776
Yu JL, Rak JW (2003) Host microenvironment in breast cancer development—inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res 5(2):83–88
Karnoub AE et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–U4
Hanna M et al (2017) Association between expression of inflammatory markers in normal breast tissue and mammographic density among premenopausal and postmenopausal women. Menopause 24(5):524–535
Bower JE et al (2011) Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 29(26):3517–3522
Janelsins MC et al (2012) Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer 20(4):831–839
Soria G et al (2011) Inflammatory mediators in breast cancer: coordinated expression of TNFalpha & IL-1beta with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 11:130
Lyon DE et al (2008) Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs Res 57(1):51–58
Premkumar VG et al (2007) Serum cytokine levels of interleukin-1 beta, -6, -8, tumour necrosis factor-alpha and vascular endothelial growth factor in breast cancer patients treated with tamoxifen and supplemented with co-enzyme Q(10), riboflavin and niacin. Basic Clin Pharmacol Toxicol 100(6):387–391
Liu L et al (2012) Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun 26(5):706–713
Mills PJ et al (2005) The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 69(1):85–96
S D (2012) Symptoms, symptom clusters and cytokine activity in women with breast cancer. PhD Thesis Royal College of Surgeons in Ireland, Dublin
Bouchard LC et al (2016) Postsurgical depressive symptoms and proinflammatory cytokine elevations in women undergoing primary treatment for breast cancer. Psychosom Med 78(1):26–37
Lyon DE, Schubert C, Taylor AG (2010) Pilot study of cranial stimulation for symptom management in breast cancer. Oncol Nurs Forum 37(4):476–483
Alfano CM et al (2012) Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol 30(12):1280–1287
Orre IJ et al (2011) Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res 71(3):136–141
Alfano CM et al (2017) Inflammatory cytokines and comorbidity development in breast cancer survivors versus noncancer controls: evidence for accelerated aging? J Clin Oncol 35(2):149
Kiecolt-Glaser JK et al (2014) Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol 32(10):1040–1049
Pierce BL et al (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27(21):3437–3444
Collado-Hidalgo A et al (2006) Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 12(9):2759–2766
Sicking I et al (2014) Prognostic influence of pre-operative C-reactive protein in node-negative breast cancer patients. PLoS ONE 9(10):e111306
Doong SH et al (2015) Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs 17(3):237–247
van Vulpen JK et al (2018) Effects of physical exercise on markers of inflammation in breast cancer patients during adjuvant chemotherapy. Breast Cancer Res Treat 168(2):421–431
Imayama I et al (2013) Weight, inflammation, cancer-related symptoms and health-related quality of life among breast cancer survivors. Breast Cancer Res Treat 140(1):159–176
Eisenberger NI et al (2010) Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 24(4):558–563
Raison CL et al (2010) Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiat 68(10):942–949
Felger JC, T MT, Haroon E, Li Z, Torresabe MA, Miller AH (2017) Inflammation-associated CNS pathways to fatigue in breast cancer patients. Brain Behav Immun 66:e39
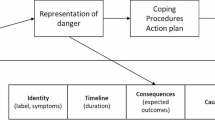
Starkweather AR et al (2013) A conceptual model of psychoneurological symptom cluster variation in women with breast cancer: bringing nursing research to personalized medicine. Curr Pharmacogenomics Person Med 11(3):224–230
National Comprehensive Cancer Network (2018) NCCN clinical practice guidelines in oncology (NCCN guidelines): cancer-related fatigue. National Comprehensive Cancer Network, Pennsylvania
Xiao C et al (2017) Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med 47(10):1733–1743
Pertl MM et al (2013) C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun 34:108–119
Abrahams HJ et al (2016) Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol 27(6):965–974
Antonella Daniele RD, Abbate I, Giotta F, Trerotoli P, Mautino A, Casamassima P, Paradiso A, Romito F, Cormio C, Carbonara MD, Mallamaci R, Mattioli V (2015) Coexistence of an imbalance of cytokines, chemokines and growth factors serum levels and symptoms of fatigue and pain in long-term breast cancer survivors. J Clin Oncol 33(15):e20626
Zick SM et al (2014) Preliminary differences in peripheral immune markers and brain metabolites between fatigued and non-fatigued breast cancer survivors: a pilot study. Brain Imaging Behav 8(4):506–516
Hampson JP et al (2015) Altered resting brain connectivity in persistent cancer related fatigue. Neuroimage Clin 8:305–313
Kotronoulas G, Wengstrom Y, Kearney N (2012) A critical review of women's sleep-wake patterns in the context of neo-/adjuvant chemotherapy for early-stage breast cancer. Breast 21(2):128–141
Koppelmans V et al (2012) Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol 30(10):1080–1086
Yang Y, Hendrix CC (2018) Cancer-related cognitive impairment in breast cancer patients: influences of psychological variables. Asia Pac J Oncol Nurs 5(3):296–306
Janelsins MC et al (2011) An update on cancer‐and chemotherapy‐related cognitive dysfunction: current status. Semin Oncol 38(3):431–438
Plassman BL et al (2008) Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 148(6):427–434
Patel SK et al (2015) Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv131
Starkweather A et al (2017) Relationships among psychoneurological symptoms and levels of C-reactive protein over 2 years in women with early-stage breast cancer. Support Care Cancer 25(1):167–176
Cheung YT et al (2015) Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol 26(7):1446–1451
Williams AM et al (2018) Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol 314:17–23
Ganz PA et al (2013) Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun 30(Suppl):S99–S108
Pomykala KL et al (2013) The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav 7(4):511–523
Kesler S et al (2013) Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun 30(Suppl):S109–S116
Orchard TS et al (2018) Diet quality, inflammation, and quality of life in breast cancer survivors: a cross-sectional analysis of pilot study data. J Acad Nutr Diet 118(4):578–588
George SM et al (2010) Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomark Prev 19(9):2220–2228
Liese AD et al (2015) The dietary patterns methods project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr 145(3):393–402
George SM et al (2014) Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J Cancer Surviv 8(4):680–687
Calder PC (2015) Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851(4):469–484
Ma Y et al (2008) Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 24(10):941–949
Aeberli I et al (2011) Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 94(2):479–485
Jameel F et al (2014) Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis 13:195
Shivappa N et al (2015) Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer 113(7):1099–1103
Guest DD, Evans EM, Rogers LQ (2013) Diet components associated with perceived fatigue in breast cancer survivors. Eur J Cancer Care (Engl) 22(1):51–59
Zick SM et al (2013) Examination of the association of diet and persistent cancer-related fatigue: a pilot study. Oncol Nurs Forum 40(1):E41–E49
Zick SM et al (2017) Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat 161(2):299–310
Huang Z et al (2018) Associations of dietary intake and supplement use with post-therapy cognitive recovery in breast cancer survivors. Breast Cancer Res Treat 171(1):189–198
Zuniga KE, Moran NE (2018) Low serum carotenoids are associated with self-reported cognitive dysfunction and inflammatory markers in breast cancer survivors. Nutrients 10(8):1111
Funding
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
George, M.A., Lustberg, M.B. & Orchard, T.S. Psychoneurological symptom cluster in breast cancer: the role of inflammation and diet. Breast Cancer Res Treat 184, 1–9 (2020). https://doi.org/10.1007/s10549-020-05808-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05808-x