Abstract
Purpose
Women with an inherited germline BRCA1 mutation have a high lifetime risk of developing breast cancer. We have previously shown that, among BRCA mutation carriers, incidence rates of breast cancer vary by country of residence.
Methods
In the current study, we prospectively calculated the cumulative and annual incidence rates of incident breast cancer, contralateral breast cancer and ipsilateral breast cancer recurrence among BRCA1 mutation carriers in Poland. Study subjects comprised a cohort of 1776 Polish women with a BRCA1 mutation who had no prior diagnosis of breast or ovarian cancer at the time of enrollment, the women were followed with a biennial follow-up by questionnaire. Women were followed for an average of 6.1 years (range 0.0–18.2) and 191 new breast cancer cases were diagnosed.
Results
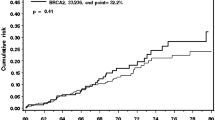
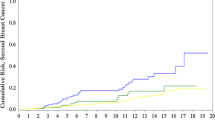
The cumulative incidence of breast cancer to age 70 was 52%. The annual risk of breast cancer was estimated at 1.78%; the maximum annual risk was observed between the ages of 30 and 65. Among the 941 women with a prior diagnosis of breast cancer, 106 women developed a contralateral breast cancer. The 20-year cumulative incidence of contralateral breast cancer was 31% and the annual rate of contralateral breast cancer was 1.96%. There were 11 recurrences among the 215 women with breast cancer (ipsilateral breast cancers). The cumulative incidence at 20 years was 17% and the annual rate of an ipsilateral recurrence was 1.03%.
Conclusion
Our findings confirm the high annual rates of early-onset incident, contralateral and recurrent breast cancer among Polish BRCA1 mutation carriers. These risk estimates are important in the context of the clinical management of unaffected women as well as in the treatment of newly diagnosed primary breast cancers and can also be used as the basis for the planning of prevention trials.




Similar content being viewed by others
References
Kuchenbaecker KB, Hopper JL, Barnes DR et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416
Lubinski J, Huzarski T, Byrski T et al (2012) The risk of breast cancer in women with a BRCA1 mutation from North America and Poland. Int J Cancer 131(1):229–234
Moller P, Maehle L, Vabo A, Clark N, Sun P, Narod SA (2013) Age-specific incidence rates for breast cancer in carriers of BRCA1 mutations from Norway. Clin Genet 83(1):88–91
Gorski B, Byrski T, Huzarski T et al (2000) Founder mutations in the BRCA1 gene in polish families with breast-ovarian cancer. Am J Hum Genet 66(6):1963–1968
Kluz T, Jasiewicz A, Marczyk E et al (2018) Frequency of BRCA1 and BRCA2 causative founder variants in ovarian cancer patients in South-East Poland. Hered Cancer Clin Pract 16:6
Cybulski C, Lubinski J, Wokolorczyk D et al (2015) Mutations predisposing to breast cancer in 12 candidate genes in breast cancer patients from Poland. Clin Genet 88(4):366–370
Narod SA, Tung N, Lubinski J et al (2014) A prior diagnosis of breast cancer is a risk factor for breast cancer in BRCA1 and BRCA2 carriers. Curr Oncol 21(2):64–68
Gronwald J, Huzarski T, Byrski T et al (2006) Direct-to-patient BRCA1 testing: the Twoj Styl experience. Breast Cancer Res Treat 100(3):239–245
Metcalfe K, Gershman S, Ghadirian P et al (2014) Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. Bmj 348:g226
Metcalfe K, Lynch HT, Ghadirian P et al (2011) Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 127(1):287–296
Pierce LJ, Phillips KA, Griffith KA et al (2010) Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 121(2):389–398
Robson ME, Chappuis PO, Satagopan J et al (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 6(1):R8–R17
Acknowledgements
We would like to acknowledge the study staff, students, and volunteers including Shana Kim, Farah Shoukat, Ellen MacDougall, Zoella Pasta, Nida Mian, Jennifer Ng, Sarah Chin, Hamida Begum, Harmeet Chaudhary, Asrafi Azmi, Shahana Nargis, Clotilde Ngwa, Mai Abdelhadi, Saiveena Penikalapati, Laavanya Somasundaram, and Hannah Horvath who helped with the data collection and data entry.
Funding
Joanne Kotsopoulos is a recipient of a Tier II Canada Research Chair. Steven A. Narod is the recipient of a Tier I Canada Research Chair. This study was supported by a Canadian Cancer Society Research Institute grant (703058) and the Peter Gilgan Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lubinski, J., Huzarski, T., Gronwald, J. et al. Age-specific risks of incident, contralateral and ipsilateral breast cancer among 1776 Polish BRCA1 mutation carriers. Breast Cancer Res Treat 174, 769–774 (2019). https://doi.org/10.1007/s10549-018-05076-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-05076-w