Abstract
Purpose
Improved therapies and imaging modalities are needed for the treatment of breast cancer brain metastases (BCBM). ANG1005 is a drug conjugate consisting of paclitaxel covalently linked to Angiopep-2, designed to cross the blood–brain barrier. We conducted a biomarker substudy to evaluate 18F-FLT–PET for response assessment.
Methods
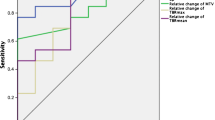
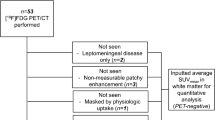
Ten patients with measurable BCBM received ANG1005 at a dose of 550 mg/m2 IV every 21 days. Before and after cycle 1, patients underwent PET imaging with 18F-FLT, a thymidine analog, retention of which reflects cellular proliferation, for comparison with gadolinium-contrast magnetic resonance imaging (Gd-MRI) in brain metastases detection and response assessment. A 20 % change in uptake after one cycle of ANG1005 was deemed significant.
Results
Thirty-two target and twenty non-target metastatic brain lesions were analyzed. The median tumor reduction by MRI after cycle 1 was −17.5 % (n = 10 patients, lower, upper quartiles: −25.5, −4.8 %) in target lesion size compared with baseline. Fifteen of twenty-nine target lesions (52 %) and 12/20 nontarget lesions (60 %) showed a ≥20 % decrease post-therapy in FLT–PET SUV change (odds ratio 0.71, 95 % CI: 0.19, 2.61). The median percentage change in SUVmax was −20.9 % (n = 29 lesions; lower, upper quartiles: −42.4, 2.0 %), and the median percentage change in SUV80 was also −20.9 % (n = 29; lower, upper quartiles: −49.0, 0.0 %). Two patients had confirmed partial responses by PET and MRI lasting 6 and 18 cycles, respectively. Seven patients had stable disease, receiving a median of six cycles.
Conclusions
ANG1005 warrants further study in BCBM. Results demonstrated a moderately strong association between MRI and 18F-FLT–PET imaging.


Similar content being viewed by others
References
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Gempt J, Bette S, Buchmann N, Ryang YM, Forschler A, Pyka T et al (2015) Volumetric analysis of F-18-FET-PET imaging for brain metastases. World Neurosurg 84(6):1790–1797
Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R et al (2008) Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol 155(2):185–197
Kurzrock R, Gabrail N, Chandhasin C, Moulder S, Smith C, Brenner A et al (2012) Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther 11(2):308–316
Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A et al (2008) Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem 106(4):1534–1544
Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R et al (2008) Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 324(3):1064–1072
Bertrand Y, Currie JC, Poirier J, Demeule M, Abulrob A, Fatehi D et al (2011) Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer 105(11):1697–1707
Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA et al (2009) Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res 26(11):2486–2494
Drappatz J, Brenner A, Wong ET, Eichler A, Schiff D, Groves MD et al (2013) Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res 19(6):1567–1576
Nowosielski M, Radbruch A (2015) The emerging role of advanced neuroimaging techniques for brain metastases. Chin Clin Oncol 4(2):23
Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF (2014) Pros and cons of current brain tumor imaging. Neuro Oncol 16(Suppl 7):vii2–vii11
Beadsmoore C, Newman D, MacIver D, Pawaroo D (2015) Positron emission tomography computed tomography: a guide for the general radiologist. Can Assoc Radiol J 66(4):332–347
Peck M, Pollack HA, Friesen A, Muzi M, Shoner SC, Shankland EG et al (2015) Applications of PET imaging with the proliferation marker [18F]-FLT. Q J Nucl Med Mol Imaging 59(1):95–104
Yamamoto Y, Nishiyama Y, Kimura N, Ishikawa S, Okuda M, Bandoh S et al (2008) Comparison of (18)F-FLT PET and (18)F-FDG PET for preoperative staging in non-small cell lung cancer. Eur J Nucl Med Mol Imaging 35(2):236–245
Halter G, Buck AK, Schirrmeister H, Aksoy E, Liewald F, Glatting G et al (2004) Lymph node staging in lung cancer using [18F]FDG-PET. Thorac Cardiovasc Surg 52(2):96–101
Cobben DC, Elsinga PH, Hoekstra HJ, Suurmeijer AJ, Vaalburg W, Maas B et al (2004) Is 18F-3′-fluoro-3′-deoxy-l-thymidine useful for the staging and restaging of non-small cell lung cancer? J Nucl Med 45(10):1677–1682
Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G et al (2003) Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med 44(9):1426–1431
Francis DL, Visvikis D, Costa DC, Arulampalam TH, Townsend C, Luthra SK et al (2003) Potential impact of [18F]3′-deoxy-3′-fluorothymidine versus [18F]fluoro-2-deoxy-d-glucose in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging 30(7):988–994
Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M et al (2007) Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 48(12):1945–1950
Cobben DC, van der Laan BF, Maas B, Vaalburg W, Suurmeijer AJ, Hoekstra HJ et al (2004) 18F-FLT PET for visualization of laryngeal cancer: comparison with 18F-FDG PET. J Nucl Med 45(2):226–231
Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ et al (2007) 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med 48(5):726–735
Choi SJ, Kim JS, Kim JH, Oh SJ, Lee JG, Kim CJ et al (2005) [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 32(6):653–659
Muzi M, Spence AM, O’Sullivan F, Mankoff DA, Wells JM, Grierson JR et al (2006) Kinetic analysis of 3′-deoxy-3′-18F-fluorothymidine in patients with gliomas. J Nucl Med 47(10):1612–1621
Chen K, Bandy D, Reiman E, Huang SC, Lawson M, Feng D et al (1998) Noninvasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J Cereb Blood Flow Metab 18(7):716–723
Pio BS, Park CK, Pietras R, Hsueh WA, Satyamurthy N, Pegram MD et al (2006) Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol 8(1):36–42
Kenny LM, Vigushin DM, Al-Nahhas A, Osman S, Luthra SK, Shousha S et al (2005) Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res 65(21):10104–10112
Trastuzumab package insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/trasgen020900lb.htm. Accessed 9 Oct 2015
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Young RJ, Sills AK, Brem S, Knopp EA (2005) Neuroimaging of metastatic brain disease Neurosurgery 57(5 Suppl):S10–S23 discusssion S1–4
Cha S (2009) Neuroimaging in neuro-oncology. Neurotherapeutics 6(3):465–477
da Cruz LCH Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 32(11):1978–1985
Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS (2003) Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res 63(18):5950–5956
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9(5):453–461
Fiegler W, Langer M, Scheer M, Kazner E (1986) Reversible computed tomographic changes following brain tumor irradiation induced by the “early-delayed reaction” after radiation. Der Radiol 26(4):206–209
Thorsen F, Fite B, Mahakian LM, Seo JW, Qin S, Harrison V et al (2013) Multimodal imaging enables early detection and characterization of changes in tumor permeability of brain metastases. J Control Release 172(3):812–822
Schiepers C, Chen W, Dahlbom M, Cloughesy T, Hoh CK, Huang SC (2007) 18F-fluorothymidine kinetics of malignant brain tumors. Eur J Nucl Med Mol Imaging 34(7):1003–1011
Bates SE (2015) Central nervous system metastasis from breast cancer. Oncologist 20(1):3–4
Lin NU, Gabrail NY, Sarantopoulos J, Schwartzberg LS, Kesari S, Bates SE et al (2014) Evaluation of CNS and peripheral antitumor activity of ANG1005 in patients with brain metastases from breast tumors and other advanced solid tumors. J Clin Oncol 32:5s (suppl; abstr 2523)
FDA approves ANG1005 for the treatment of glioblastoma multiforme. http://angiochem.com/angiochem%E2%80%99s-ang1005-received-orphan-drug-designation-fda-treatment-glioblastoma-multiform. Accessed 6 Feb 2016
Lim E, Lin NU (2014) Updates on the management of breast cancer brain metastases. Oncology 28(7):572–578
Paclitaxel (Taxol) package insert data. https://www.medicines.org.uk/emc/PIL.25823.latest.pdf. Accessed 19 Aug 2015
Acknowledgments
We would like to thank all the patients, their families, the research, and allied health professional staff in the National Cancer Institute for their valuable contributions to this study. ANG1005 was provided by Angiochem, and representatives from the company reviewed the manuscript. None of the authors have a financial relationship with Angiochem or have other relevant conflicts of interest to disclose.
Author contributions
Both Susan E. Bates and Antonio T. Fojo had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any relevant disclosures.
Rights and permissions
About this article
Cite this article
O’Sullivan, C.C., Lindenberg, M., Bryla, C. et al. ANG1005 for breast cancer brain metastases: correlation between 18F-FLT–PET after first cycle and MRI in response assessment. Breast Cancer Res Treat 160, 51–59 (2016). https://doi.org/10.1007/s10549-016-3972-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3972-z