Abstract
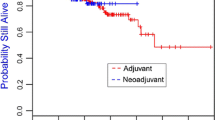
The purpose of this study was to assess pathological complete response and whether it serves a surrogate for survival among patients receiving neo-adjuvant doxorubicin–cyclophosphamide followed by paclitaxel for triple-negative breast cancer with respect to BRCA1 mutation status. From a neo-adjuvant systemic therapy database of 588 breast cancer cases, 80 triple-negative cases who had undergone BRCA genotyping were identified. Logistic regression model was fitted to examine the association between BRCA1 status and pathological complete response. Survival outcomes were evaluated using Kaplan–Meier method, differences between study groups calculated by log-rank test. Thirty-four BRCA1 carriers and 43 non-carriers were identified. The BRCA1 carriers had pathological complete response rate of 68 % compared with 37 % among non-carriers, p = 0.01. Yet this did not translate into superior survival for BRCA1 carriers compared with non-carriers. No difference in relapse-free survival were noted among those with or without pathological complete response in BRCA1 carriers regardless of pathological complete response status (Log-rank p = 0.25), whereas in the non-carrier cohort, relapse-free survival was superior for those achieving pathological complete response (Log-rank p < 0.0001). Response to neo-adjuvant systemic therapy differed in BRCA1-associated triple-negative breast cancer compared with triple-negative non-carriers, with a higher rate of pathological complete response. However, compared with non-carrier triple-negative breast cancer, pathological complete response was not a surrogate for superior relapse-free survival in BRCA1 patients. Future studies using specific chemotherapy regimens may provide further improvements in outcomes.




Similar content being viewed by others
References
Abbott DW, Freeman ML, Holt JT (1998) Double-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cells. J Natl Cancer Inst 90:978–985
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, Litton JK, Valero V, Meric-Bernstam F, Hortobagyi GN, Albarracin C (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29:3739–3746. doi:10.1200/JCO.2011.35.2682
Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN, Arun BK (2008) Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 26:4282–4288. doi:10.1200/JCO.2008.16.6231
Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK, Lin E, Albarracin CT, Meric-Bernstam F, Gonzalez-Angulo AM, Hortobagyi GN, Arun BK (2011) Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat 130:145–153. doi:10.1007/s10549-011-1711
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Dent R, Lubinski J, Narod S (2010) Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28:375–379. doi:10.1200/JCO.2008.20.7019
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Narod SA, Lubinski J, Polish Hereditary Breast Cancer C (2008) Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat 108:289–296. doi:10.1007/s10549-007-9600-1
Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M (2011) Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi Women. Breast Cancer Res Treat 129:185–190. doi:10.1007/s10549-011-1433-2
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474. doi:10.1245/s10434-010-0985-4
Ellis P, Smith I, Ashley S, Walsh G, Ebbs S, Baum M, Sacks N, McKinna J (1998) Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer. J Clin Oncol 16:107–114
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917–921. doi:10.1038/nature03445
Fasching PA, Eidtmann H, Tesch H, Untch M, Hilfrich J, Schem C, Rezai M, Gerber B, Costa SD, Blohmer JU, Fehm TN, Huober J, Liedtke C, Müller V, Nekljudova V, Weber K, Rack B, Rübner M, Wang L, Ingle JN, Weinshilboum RM, von Minckwitz G, Couch F (2015) BRCA mutations, therapy response and prognosis in the neoadjuvant GeparQuinto study In: San Antonio Breast Cancer Symposium. San Antonio
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, O’Malley FP, Milne RL, Andrulis IL, Friedlander ML, Southey MC, Apicella C, Giles GG, Longacre TA (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30:19–26. doi:10.1200/JCO.2010.33.0068
Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA (1999) The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 64:963–970
Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, Vidaurre T, Holmes F, Souchon E, Wang H, Martin M, Cotrina J, Gomez H, Hubbard R, Chacon JI, Ferrer-Lozano J, Dyer R, Buxton M, Gong Y, Wu Y, Ibrahim N, Andreopoulou E, Ueno NT, Hunt K, Yang W, Nazario A, DeMichele A, O’Shaughnessy J, Hortobagyi GN, Symmans WF (2011) A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA 305:1873–1881. doi:10.1001/jama.2011.593
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka M, Morawiec Z, Mierzwa T, Janiszewska H, Kilar E, Marczyk E, Kozak-Klonowska B, Siolek M, Surdyka D, Wisniowski R, Posmyk M, Sun P, Lubinski J, Narod SA (2013) Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 31:3191–3196. doi:10.1200/JCO.2012.45.3571
Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W, Lebeau A, Loibl S, Miller W, Seeber S, Semiglazov V, Smith R, Souchon R, Stearns V, Untch M, von Minckwitz G (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24:1940–1949. doi:10.1200/JCO.2005.02.6187
King MC, Marks JH, Mandell JB, New York Breast Cancer Study G (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646. doi:10.1126/science.1088759
Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, Binkley SM, Ames FC, Feig BW, Ross MI, Hortobagyi GN, Singletary SE (1998) Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg 176:502–509
Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF (2002) The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol 20:2310–2318
Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, Ursin G (2011) Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J Clin Oncol 29:4373–4380. doi:10.1200/JCO.2010.33.6446
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281. doi:10.1200/JCO.2007.14.4147
Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, Narod SA (2007) Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med 357:115–123. doi:10.1056/NEJMoa070608
Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Begin LR, Offit K, Foulkes WD (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. BCR 6:R8–R17. doi:10.1186/bcr658
Rohlfs EM, Learning WG, Friedman KJ, Couch FJ, Weber BL, Silverman LM (1997) Direct detection of mutations in the breast and ovarian cancer susceptibility gene BRCA1 by PATHOLOGICAL COMPLETE RESPONSE-mediated site-directed mutagenesis. Clin Chem 43:24–29
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA (2008) Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 14:8010–8018. doi:10.1158/1078-0432.CCR-08-1208
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, Golshan M, Bellon JR, Collyar D, Hahn OM, Carey LA, Hudis CA, Winer EP (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33:13–21. doi:10.1200/JCO.2014.57.0572
Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA (1997) The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336:1401–1408. doi:10.1056/NEJM199705153362001
Tutt A (2014) TNT: a randomized phase III trial of carboplatin compared with docetaxel for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer. In: San Antonio Breast Cancer Symposium
Tutt AN, Lord CJ, McCabe N, Farmer H, Turner N, Martin NM, Jackson SP, Smith GC, Ashworth A (2005) Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb Symp Quant Biol 70:139–148. doi:10.1101/sqb.2005.70.012
Veronesi A, de Giacomi C, Magri MD, Lombardi D, Zanetti M, Scuderi C, Dolcetti R, Viel A, Crivellari D, Bidoli E, Boiocchi M (2005) Familial breast cancer: characteristics and outcome of BRCA 1-2 positive and negative cases. BMC Cancer 5:70. doi:10.1186/1471-2407-5-70
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Kummel S, Paepke S, Schneeweiss A, Untch M, Zahm DM, Mehta K, Loibl S (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31:3623–3630. doi:10.1200/JCO.2012.45.0940
von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, Zahm DM, Kummel S, Eidtmann H, Klare P, Huober J, Costa S, Tesch H, Hanusch C, Hilfrich J, Khandan F, Fasching PA, Sinn BV, Engels K, Mehta K, Nekljudova V, Untch M (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15:747–756. doi:10.1016/S1470-2045(14)70160-3
von Minckwitz G, Untch M, Nuesch E, Loibl S, Kaufmann M, Kummel S, Fasching PA, Eiermann W, Blohmer JU, Costa SD, Mehta K, Hilfrich J, Jackisch C, Gerber B, du Bois A, Huober J, Hanusch C, Konecny G, Fett W, Stickeler E, Harbeck N, Muller V, Juni P (2011) Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 125:145–156. doi:10.1007/s10549-010-1228
Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham-Hawkins D, Hamel N, Di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet JS, Narod S (1999) Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91:1241–1247
Acknowledgments
The authors thank Karen Gelmon for her guidance, input, and review of the manuscript.
Author’s contributions
S Paluch-Shimon, E. Friedman, R. Berger, M. Papa, M. Dadiani, and B. Kaufman contributed to conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, and revising it critically for important intellectual content. M. Shabtai, D. Zippel, M. Gutman, N. Friedman A. Yosepovich, and T. Modiano significantly contributed to acquisition of data and revising manuscript. T. Golan and R. Catane contributed to drafting the manuscript and revising it critically. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Funding
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paluch-Shimon, S., Friedman, E., Berger, R. et al. Neo-adjuvant doxorubicin and cyclophosphamide followed by paclitaxel in triple-negative breast cancer among BRCA1 mutation carriers and non-carriers. Breast Cancer Res Treat 157, 157–165 (2016). https://doi.org/10.1007/s10549-016-3800-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3800-5