Abstract
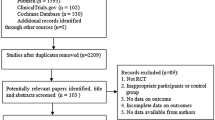
Compared to tamoxifen, the efficacy and side effects of toremifene in adjuvant endocrine therapy for breast cancer were not very clear. This meta-analysis was conducted to give a more precise estimation of the efficacy and severe side effects of toremifene given in the adjuvant setting in comparison to tamoxifen. The electronic database PubMed was searched for randomized trials comparing toremifene with tamoxifen as adjuvant therapies. Four randomized trials published in three articles were eligible, including 1,890 pooled cases treated with toremifene and 1,857 cases treated with tamoxifen. Compared to patients in tamoxifen group, patients in toremifene group did not have a significantly different overall survival rate (risk ratio (RR): 1.07, 95% confidence interval (CI): 0.97–1.19, P = 0.994 for heterogeneity) or a disease-free survival (DFS) rate (RR: 1.05, 95% CI: 0.95–1.17, P = 0.431 for heterogeneity) at the end of the follow-up time. The rates of thromboembolic events in toremifene group, including deep vein thrombosis (odds ratio (OR): 0.68, 95% CI: 0.40–1.17, P = 0.926 for heterogeneity), cerebrovascular accident (OR: 0.59, 95% CI: 0.32–1.09, P = 0.438 for heterogeneity), and pulmonary embolism (OR: 0.91, 95% CI: 0.42–2.01, P = 0.618 for heterogeneity), were not significantly different from those in tamoxifen group. The rates of endometrial polyps and endometrial cancer between the two groups were almost the same. This meta-analysis suggested that toremifene was as effective as tamoxifen in the adjuvant setting for both perimenopausal and postmenopausal breast cancer patients with similar severe adverse effects to tamoxifen. Toremifene was a convincing and safe change for tamoxifen in adjuvant endocrine therapy.



Similar content being viewed by others
References
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339(22):1609–1618. doi:10.1056/NEJM199811263392207
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9(1):45–53. doi:10.1016/S1470-2045(07)70385-6
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25(5):486–492. doi:10.1200/JCO.2006.08.8617
Winer EP, Hudis C, Burstein HJ, Chlebowski RT, Ingle JN, Edge SB, Mamounas EP, Gralow J, Goldstein LJ, Pritchard KI, Braun S, Cobleigh MA, Langer AS, Perotti J, Powles TJ, Whelan TJ, Browman GP (2002) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol 20(15):3317–3327
Kallio S, Kangas L, Blanco G, Johansson R, Karjalainen A, Perila M, Pippo I, Sundquist H, Sodervall M, Toivola R (1986) A new triphenylethylene compound, Fc-1157a. I. Hormonal effects. Cancer Chemother Pharmacol 17(2):103–108
Pyrhonen S, Ellmen J, Vuorinen J, Gershanovich M, Tominaga T, Kaufmann M, Hayes DF (1999) Meta-analysis of trials comparing toremifene with tamoxifen and factors predicting outcome of antiestrogen therapy in postmenopausal women with breast cancer. Breast Cancer Res Treat 56(2):133–143
Holli K, Valavaara R, Blanco G, Kataja V, Hietanen P, Flander M, Pukkala E, Joensuu H (2000) Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer Finnish Breast Cancer Group. J Clin Oncol 18(20):3487–3494
Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, Simoncini E, Castiglione-Gertsch M, Coates AS, Goldhirsch A (2004) Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12–93 and 14–93. Ann Oncol 15(12):1749–1759. doi:10.1093/annonc/mdh463
Lewis JD, Chagpar AB, Shaughnessy EA, Nurko J, McMasters K, Edwards MJ (2010) Excellent outcomes with adjuvant toremifene or tamoxifen in early stage breast cancer. Cancer 116(10):2307–2315. doi:10.1002/cncr.24940
Hirsimaki P, Aaltonen A, Mantyla E (2002) Toxicity of antiestrogens. Breast J 8(2):92–96
Liu X, Wang Z, Yu J, Lei G, Wang S (2010) Three polymorphisms in interleukin-1beta gene and risk for breast cancer: a meta-analysis. Breast Cancer Res Treat 124(3):821–825. doi:10.1007/s10549-010-0910-3
Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26(1):53–77. doi:10.1002/sim.2528
Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A (2010) Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10:337. doi:10.1186/1471-2407-10-337
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127(9):820–826
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Weinshilboum RM, Fritcher EG, Nibbe AM, Desta Z, Nguyen A, Flockhart DA, Perez EA, Ingle JN (2007) The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101(1):113–121. doi:10.1007/s10549-006-9428-0
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5(1):6–13. doi:10.1038/sj.tpj.6500285
Carpenter R (2008) Choosing early adjuvant therapy for postmenopausal women with hormone-sensitive breast cancer: aromatase inhibitors versus tamoxifen. Eur J Surg Oncol 34(7):746–755. doi:10.1016/j.ejso.2008.01.011
Saarto T, Blomqvist C, Ehnholm C, Taskinen MR, Elomaa I (1996) Antiatherogenic effects of adjuvant antiestrogens: a randomized trial comparing the effects of tamoxifen and toremifene on plasma lipid levels in postmenopausal women with node-positive breast cancer. J Clin Oncol 14(2):429–433
Shibutani S, Ravindernath A, Terashima I, Suzuki N, Laxmi YR, Kanno Y, Suzuki M, Apak TI, Sheng JJ, Duffel MW (2001) Mechanism of lower genotoxicity of toremifene compared with tamoxifen. Cancer Res 61(10):3925–3931
Shibutani S, Ravindernath A, Suzuki N, Terashima I, Sugarman SM, Grollman AP, Pearl ML (2000) Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis 21(8):1461–1467
Homesley HD, Shemano I, Gams RA, Harry DS, Hickox PG, Rebar RW, Bump RC, Mullin TJ, Wentz AC, O’Toole RV et al (1993) Antiestrogenic potency of toremifene and tamoxifen in postmenopausal women. Am J Clin Oncol 16(2):117–122
di Salle E, Zaccheo T, Ornati G (1990) Antiestrogenic and antitumor properties of the new triphenylethylene derivative toremifene in the rat. J Steroid Biochem 36(3):203–206
Acknowledgments
We are grateful to Dr. Ji-Fu Wei (Clinical Experiment Center, the First Affiliated Hospital with Nanjing Medical University) for critical discussion in our study. The authors thank Dr. Tian-Song Xia for his help in revising the manuscript. This study was supported in part by the National Natural Science Foundation of China (81071753), the Six Kinds of Outstanding Talent Foundation of Jiangsu Province (06-B-069 and 2009, To Qiang Ding), the Science and Education for Health Foundation of Jiangsu Province (RC2007054), the Natural Science Foundation of Jiangsu Province (BK2008476, BK2009438, and BK2010581), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU (IRT-008), and A project Funded by the Priority Academic Program Development of Jiangsu higher Education Institutions (PAPD).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wen-Bin Zhou and Qiang Ding contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhou, WB., Ding, Q., Chen, L. et al. Toremifene is an effective and safe alternative to tamoxifen in adjuvant endocrine therapy for breast cancer: results of four randomized trials. Breast Cancer Res Treat 128, 625–631 (2011). https://doi.org/10.1007/s10549-011-1556-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1556-5