Abstract
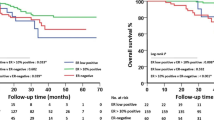
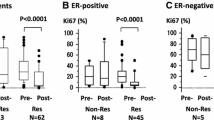
The NICE trial was designed to evaluate the possible benefits of adding epidermal growth factor receptor targeted therapy to neoadjuvant chemotherapy in patients with estrogen receptor α (ER) negative and operable breast cancer. Preclinical data have suggested that signalling through the ErbB receptors or downstream effectors may repress ER expression. Here the authors investigated whether gefitinib, given neoadjuvant in combination with epirubicin and cyclophosphamide (EC), could restore ER expression. Eligible patients in the NICE trial were women with unilateral, primary operable, ER negative invasive breast cancer ≥2 cm. Material from patients randomized and completing treatment (four cycles of neoadjuvant EC plus 12 weeks of either gefitinib or placebo) in the NICE trial having available ER status both at baseline and after neoadjuvant treatment were eligible for this study. Tumors with indication of changed ER phenotype (based on collected pathology reports) were immunohistochemically reassessed centrally. 115 patients were eligible for this study; 59 patients in the gefitinib group and 56 patients in the placebo group. Five (4.3%) of 115 tumors changed ER phenotype from negative to positive. A change was seen in three patients in the gefitinib (5.1%) and in two patients in the placebo (3.6%) group with a difference of 1.51% (95% CI, −6.1–9.1). Results of the NICE trial have been reported previously. Post-operative reassessment of ER expression changed the assessment of ER status in a small but significant fraction of patients and should, whenever possible, be performed following neoadjuvant chemotherapy for ER negative breast cancer. Gefitinib did not affect the reversion rate of ER negative tumors.

Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Revillion F, Bonneterre J, Peyrat JP (1998) ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer 34(6):791–808
Klijn JG, Berns PM, Schmitz PI, Foekens JA (1992) The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev 13(1):3–17
Sainsbury JR, Farndon JR, Sherbet GV, Harris AL (1985) Epidermal-growth-factor receptors and oestrogen receptors in human breast cancer. Lancet 1(8425):364–366
Sonne-Hansen K, Norrie IC, Emdal KB, Benjaminsen RV, Frogne T, Christiansen IJ et al (2010) Breast cancer cells can switch between estrogen receptor alpha and ErbB signaling and combined treatment against both signaling pathways postpones development of resistance. Breast Cancer Res Treat 121(3):601–613
Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV et al (2009) Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat 114(2):263–275
Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME et al (2003) Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144(3):1032–1044
Pancholi S, Lykkesfeldt AE, Hilmi C, Banerjee S, Leary A, Drury S et al (2008) ERBB2 influences the subcellular localization of the estrogen receptor in tamoxifen-resistant MCF-7 cells leading to the activation of AKT and RPS6KA2. Endocr Relat Cancer 15(4):985–1002
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H et al (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96(12):926–935
Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L et al (2006) A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA 103(20):7795–7800
Ghayad SE, Vendrell JA, Larbi SB, Dumontet C, Bieche I, Cohen PA (2010) Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3 K/Akt signaling pathways. Int J Cancer 126(2):545–562
Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA et al (2010) Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res 16(5):1486–1497
Brinkman JA, El-Ashry D (2009) ER re-expression and re-sensitization to endocrine therapies in ER-negative breast cancers. J Mammary Gland Biol Neoplasia 14(1):67–78
Bayliss J, Hilger A, Vishnu P, Diehl K, El-Ashry D (2007) Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res 13(23):7029–7036
Munzone E, Curigliano G, Rocca A, Bonizzi G, Renne G, Goldhirsch A et al (2006) Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res 8(1):R4
Bernsdorf M, Ingvar C, Jorgensen L, Tuxen MK, Jakobsen EH, Saetersdal A et al (2011) Effect of adding gefitinib to neoadjuvant chemotherapy in estrogen receptor negative early breast cancer in a randomized phase II trial. Breast Cancer Res Treat 126(2):463–470
Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB et al (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol 25(25):3846–3852
Massarweh S, Schiff R (2007) Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res 13(7):1950–1954
Taucher S, Rudas M, Gnant M, Thomanek K, Dubsky P, Roka S et al (2003) Sequential steroid hormone receptor measurements in primary breast cancer with and without intervening primary chemotherapy. Endocr Relat Cancer 10(1):91–98
Arslan C, Sari E, Aksoy S, Altundag K (2011) Variation in hormone receptor and HER-2 status between primary and metastatic breast cancer: review of the literature. Expert Opin Ther Targets 15(1):21–30
Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V et al (2005) Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol 23(23):5323–5333
Ciardiello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358(11):1160–1174
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY et al (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 21(12):2237–2246
Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP et al (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290(16):2149–2158
Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis GA, Cohen C (2010) Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: a tissue microarray-based study. Appl Immunohistochem Mol Morphol 18(5):433–441
Acknowledgments
The NICE trial and this study was sponsered by Astra Zeneca. The authors would like to thank the following centers for collaboration: Sweden: Malmö Hospital and Lund University Hospital. Norway: Trondheim Hospital and Rikshospitalet-Radiumhospitalet Oslo. Denmark: Herlev Hospital, Aarhus University Hospital, Odense University Hospital, Vejle Hospital, Roskilde Hospital, Naestved Hospital and Ringsted Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernsdorf, M., Balslev, E., Lykkesfeldt, A.E. et al. Value of post-operative reassessment of estrogen receptor α expression following neoadjuvant chemotherapy with or without gefitinib for estrogen receptor negative breast cancer. Breast Cancer Res Treat 128, 165–170 (2011). https://doi.org/10.1007/s10549-011-1535-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1535-x