Abstract
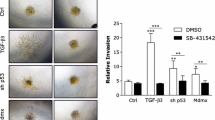
TIMP-1 (Tissue inhibitor of matrix metalloproteinase-1) is typically associated with inhibition of matrix metalloproteinases (MMP) induced invasion. However, TIMP-1 is overexpressed in many malignancies and is associated with poor prognosis in breast cancer. The mechanisms by which TIMP-1 promotes tumorigenesis are unclear. Reduced levels of TIMP-1 mediated by shRNA in MDA-MB-231 breast cancer cells had no effect on cellular physiology in vitro or tumor growth in SCID mice compared to vector control MDA-MB-231 cells. However, overexpression of TIMP-1 in MDA-MB-231 cells resulted in inhibition of cell invasion and enhanced phosphorylation of p38 MAPK and AKT in vitro. Additionally, treatment of parental MDA-MB-231 cells with purified TIMP-1 protein led to activation of p38 MAPK and MKK 3/6. cDNA array analysis demonstrated that high expression of TIMP-1 in MDA-MB-231 cells resulted in alterations in expression of approximately 200 genes, 1.5 fold or greater compared to vector control cells (P < 0.1). Real-time RT-PCR confirmed changes in expression of several genes associated with cancer progression including DAPK1, FGFR4 and MAPK13. In vivo, high TIMP-1 expression induced tumor growth in SCID mice compared to vector control cells and increased tumor vessel density. Affymetrix array analysis of vector control and TIMP-1 MDA-MB-231 xenograft tumors revealed that TIMP-1 altered expression of approximately 600 genes in vivo, including MMP1, MMP13, S100A14, S100P, Rab25 and ID4. These combined observations suggest that the effects of TIMP-1 differ significantly in a 2-D environment compared to the 3-D environment and that TIMP-1 stimulates tumor growth.





Similar content being viewed by others
References
Guedez L, McMarlin AJ, Kingma DW, Bennett TA, Stetler-Stevenson M, Stetler-Stevenson WG (2001) Tissue inhibitor of metalloproteinase-1 alters the tumorigenicity of Burkitt’s lymphoma via divergent effects on tumor growth and angiogenesis. Am J Pathol 158:1207–1215
Kong Y, Poon R, Nadesan P, Di Muccio T, Fodde R, Khokha R et al (2004) Matrix metalloproteinase activity modulates tumor size, cell motility, and cell invasiveness in murine aggressive fibromatosis. Cancer Res 64:5795–5803. doi:10.1158/0008-5472.CAN-03-3112
Rigg AS, Lemoine NR (2001) Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther 8:869–878. doi:10.1038/sj.cgt.7700387
Khokha R, Waterhouse P, Yagel S, Lala PK, Overall CM, Norton G et al (1989) Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science 243:947–950. doi:10.1126/science.2465572
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174. doi:10.1038/nrc745
McCarthy K, Maguire T, McGreal G, McDermott E, O’Higgins N, Duffy MJ (1999) High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer 84:44–48. doi :10.1002/(SICI)1097-0215(19990219)84:1<44::AID-IJC9>3.0.CO;2-P
Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O (1997) High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res 3:1623–1628
Nakopoulou L, Giannopoulou I, Stefanaki K, Panayotopoulou E, Tsirmpa I, Alexandrou P et al (2002) Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol 197:307–313. doi:10.1002/path.1129
Schrohl AS, Christensen IJ, Pedersen AN, Jensen V, Mouridsen H, Murphy G et al (2003) Tumor tissue concentrations of the proteinase inhibitors tissue inhibitor of metalloproteinases-1 (TIMP-1) and plasminogen activator inhibitor type 1 (PAI-1) are complementary in determining prognosis in primary breast cancer. Mol Cell Proteomics 2:164–172. doi:10.1074/mcp.M300019-MCP200
Jiang Y, Goldberg ID, Shi YE (2002) Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 21:2245–2252. doi:10.1038/sj.onc.1205291
Wurtz SO, Schrohl AS, Sorensen NM, Lademann U, Christensen IJ, Mouridsen H et al (2005) Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer 12:215–227. doi:10.1677/erc.1.00719
Gasson JC, Golde DW, Kaufman SE, Westbrook CA, Hewick RM, Kaufman RJ et al (1985) Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature 315:768–771. doi:10.1038/315768a0
Bertaux B, Hornebeck W, Eisen AZ, Dubertret L (1991) Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol 97:679–685. doi:10.1111/1523-1747.ep12483956
Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K (1992) Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett 298:29–32. doi:10.1016/0014-5793(92)80015-9
Porter JF, Shen S, Denhardt DT (2004) Tissue inhibitor of metalloproteinase-1 stimulates proliferation of human cancer cells by inhibiting a metalloproteinase. Br J Cancer 90:463–470. doi:10.1038/sj.bjc.6601533
Taube ME, Liu XW, Fridman R, Kim HR (2006) TIMP-1 regulation of cell cycle in human breast epithelial cells via stabilization of p27(KIP1) protein. Oncogene 25:3041–3048. doi:10.1038/sj.onc.1209336
Yoshiji H, Harris SR, Raso E, Gomez DE, Lindsay CK, Shibuya M et al (1998) Mammary carcinoma cells over-expressing tissue inhibitor of metalloproteinases-1 show enhanced vascular endothelial growth factor expression. Int J Cancer 75:81–87. doi :10.1002/(SICI)1097-0215(19980105)75:1<81::AID-IJC13>3.0.CO;2-G
Akahane T, Akahane M, Shah A, Connor CM, Thorgeirsson UP (2004) TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res 301:158–167. doi:10.1016/j.yexcr.2004.08.002
Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR (2002) Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115:3427–3438
Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL (1995) Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol 177:275–283. doi:10.1002/path.1711770310
Hansen S, Sorensen FB, Vach W, Grabau DA, Bak M, Rose C (2004) Microvessel density compared with the Chalkley count in a prognostic study of angiogenesis in breast cancer patients. Histopathology 44:428–436. doi:10.1111/j.1365-2559.2004.01848.x
Liu XW, Bernardo MM, Fridman R, Kim HR (2003) Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem 278:40364–40372. doi:10.1074/jbc.M302999200
Pramanik R, Qi X, Borowicz S, Choubey D, Schultz RM, Han J et al. (2003). p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J Biol Chem 278:4831–4839. doi:10.1074/jbc.M207732200
Porter JF, Sharma S, Wilson DL, Kappil MA, Hart RP, Denhardt DT (2005) Tissue inhibitor of metalloproteinases-1 stimulates gene expression in MDA-MB-435 human breast cancer cells by means of its ability to inhibit metalloproteinases. Breast Cancer Res Treat 94:185–193. doi:10.1007/s10549-005-7728-4
Bialik S, Kimchi A (2004) DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. Semin Cancer Biol 14:283–294. doi:10.1016/j.semcancer.2004.04.008
Jaakkola S, Salmikangas P, Nylund S, Partanen J, Armstrong E, Pyrhonen S et al (1993) Amplification of fgfr4 gene in human breast and gynecological cancers. Int J Cancer 54:378–382. doi:10.1002/ijc.2910540305
Johnston CL, Cox HC, Gomm JJ, Coombes RC (1995) bFGF and aFGF induce membrane ruffling in breast cancer cells but not in normal breast epithelial cells: FGFR-4 involvement. Biochem J 306(Pt 2):609–616
Koziczak M, Hynes NE (2004) Cooperation between fibroblast growth factor receptor-4 and ErbB2 in regulation of cyclin D1 translation. J Biol Chem 279:50004–50011. doi:10.1074/jbc.M404252200
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25:9–34. doi:10.1007/s10555-006-7886-9
Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26. doi:10.1002/jcp.20948
Feltes CM, Kudo A, Blaschuk O, Byers SW (2002) An alternatively spliced cadherin−11 enhances human breast cancer cell invasion. Cancer Res 62:6688–6697
Kondo S, Lu Y, Debbas M, Lin AW, Sarosi I, Itie A et al (2003) Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1). Proc Natl Acad Sci USA 100:5431–5436. doi:10.1073/pnas.0530308100
Sharma G, Mirza S, Prasad CP, Srivastava A, Gupta SD, Ralhan R (2007) Promoter hypermethylation of p16INK4A, p14ARF, CyclinD2 and Slit2 in serum and tumor DNA from breast cancer patients. Life Sci 80:1873–1881. doi:10.1016/j.lfs.2007.02.026
Dallol A, Da Silva NF, Viacava P, Minna JD, Bieche I, Maher ER et al (2002) SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res 62:5874–5880
Schmid BC, Rezniczek GA, Fabjani G, Yoneda T, Leodolter S, Zeillinger R (2007) The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res Treat 106:333–342. doi:10.1007/s10549-007-9504-0
Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T et al (2003) Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer 98:18–23. doi:10.1002/cncr.11482
Woodfield GW, Horan AD, Chen Y, Weigel RJ (2007) TFAP2C controls hormone response in breast cancer cells through multiple pathways of estrogen signaling. Cancer Res 67:8439–8443. doi:10.1158/0008-5472.CAN-07-2293
Martin MD, Matrisian LM (2007) The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev 26:717–724. doi:10.1007/s10555-007-9089-4
Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C et al (2003) Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 3:589–601. doi:10.1016/S1535-6108(03)00133-8
Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J (1999) The generation of endostatin is mediated by elastase. Cancer Res 59:6052–6056
Dasse E, Bridoux L, Baranek T, Lambert E, Salesse S, Sowa ML et al (2007) Tissue inhibitor of metalloproteinase-1 promotes hematopoietic differentiation via caspase−3 upstream the MEKK1/MEK6/p38alpha pathway. Leukemia 21:595–603
Wang T, Yamashita K, Iwata K, Hayakawa T (2002) Both tissue inhibitors of metalloproteinases-1 (TIMP-1) and TIMP-2 activate Ras but through different pathways. Biochem Biophys Res Commun 296:201–205. doi:10.1016/S0006-291X(02)00741-6
Jung KK, Liu XW, Chirco R, Fridman R, Kim HR (2006) Identification of CD63 as a tissue inhibitor of metalloproteinase−1 interacting cell surface protein. Embo J 25:3934–3942
Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S et al (2007) Tissue inhibitor of metalloproteinases−1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res 67:8615–8623. doi:10.1158/0008-5472.CAN-07-0232
Acknowledgements
The work was funded through the Department of Defense, Grant # BC020675 (JAC) and the National Institute of Health, NRSA, Fellowship #CA112714 (RLHB).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Affymetrix cDNA array analysis of MDA-MB-231 pcDNA pool #1 and TIMP-1 pool #1 breast cancer cells in monolayer culture (XLS 51 kb)
Supplementary Table 2
Affymetrix cDNA array analysis of MDA-MB-231 pcDNA pool #1 and TIMP-1 pool #1 MDA-MB-231 breast cancer xenografts (XLS 148 kb)
Rights and permissions
About this article
Cite this article
Bigelow, R.L.H., Williams, B.J., Carroll, J.L. et al. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat 117, 31–44 (2009). https://doi.org/10.1007/s10549-008-0170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0170-7