Abstract
Background
The Van Nuys Prognostic Index (VNPI) is a simple score for predicting the risk of local recurrence (LR) in patients with Ductal Carcinoma In Situ (DCIS) conservatively treated. This score combines three independent predictors of Local Recurrence. The VNPI has recently been updated with the addition of age as a fourth parameter into the scoring system (University of Southern California/ VNPI).
Patients and methods
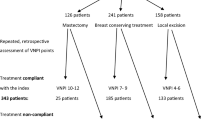
Our database consisted of 408 women with DCIS. Applying the USC/VNPI we reviewed retrospectively 259 patients who were treated with breast conserving surgery with or without radiotherapy (RT). Of these patients 63.5% had a low VNPI score, 32% intermediate and 4.5% a high score. In the low score group, the majority of the patients underwent Conservative Surgery (CS) without RT while in the intermediate group, almost half of the patients received RT. Eighty-three percent (83%) of the patients with high VNPI were treated with Conservative Surgery plus RT. Nodal assessment by Sentinel Lymph Node Biopsy was obtained in 32 patients since 2002.
Results
Twenty-one Local Recurrences were observed (8%) with a mean follow up of 130 months: sixteen were invasive. No statistically significant differences in Disease Free Survival were reached in all groups of VNPI score between patients treated with Conservative Surgery or Conservative Surgery plus RT. However it was noted that the higher the VNPI score, the lower was the risk of local recurrence in the group treated additionally with RT, even though it was not statistically significant. Further analysis included those patients treated with Conservative Surgery alone and followed up. Disease-free survival (DFS) at 10 years was 94% with low VNPI and 83% in both intermediate and high score (P < 0.05). No significant differences were observed in the subgroups of VNPI. The Local Relapse rate after Conservative Surgery alone, increased with tumor size, margin width, and pathology classification (P < 0,05), while age was not found to be a significant factor. Lesions with only mammographic appearances are associated with lower DFS but it did not reach significance (P = ns), while assumption of estrogenic hormones and familial history of breast cancer are significant factors associated with a higher risk of local recurrence. After multivariate analysis including seven clinical and pathological factors, the only significant predictors of local recurrence remained margin width of surgical excision, previous therapy with estrogens (contraceptives or Hormone Replacement Therapy) and the Van Nuys pathologic classification. The overall survival breast cancer specific was 99% and no differences were observed between groups (P = ns). The comparison of patients treated with a total mastectomy and those conservatively treated showed a significantly better local relapse free survival rate obtained with mastectomy (98.2% vs. 89.7% at 10 years P = 0.02). However, the overall cause-specific survival did not prove any better outcome (98.7% in both groups). Of the 32 patients who underwent a Sentinel Lymph Node Biopsy, four were found to have micrometastases and all of them had a previous Directional Vacuum Assisted Biopsy.
Conclusions
Although in our series there is not a significant difference in LR rates by the parameter of age, the new USC/VNPI is still a simple and reliable scoring system for therapeutic management of DCIS. We did not find any statistically significant advantage in groups treated with the addition of RT. Obtaining wide surgical margins appears to be the strongest prognostic factor for local recurrence, regardless of other pathological factors or the addition of adjuvant radiation therapy. However, only prospective randomized studies can precisely predict the risk of LR of conservatively treated DCIS. The clinical significance of Sentinel Lymph Nodes micrometastases Immuno-Histo-Chemistry-detected found in DCIS patients remains uncertain. However, we hypothesize that the anatomical disruption after preoperative biopsy procedures increases the likelihood of epithelial cell displacement and the frequency of IHC-positive Sentinel Lymph Nodes, both of which are directly proportional to the degree of manipulation.













Similar content being viewed by others
References
Sunshine JA et al (1985) Breast carcinoma in situ. A retrospective review of 112 cases with a minimum 10 year follow up. Am J Surg 150:44–51
Lennington WJ et al (1994) DCIS of the breast. Heterogeneity of individual lesions. Cancer 73:118–124
Silverstein MJ (2003) Current controversies in DCIS: summary from the Lynn Sage Breast Cancer Symposium. J Am Coll Surg 197(1):115–118
Ernster VL et al (1996) Incidence and treatment for DCIS of the breast. JAMA 275:913–918
Greenlee RT et al (2000) Cancer statistics 2000 CA. Cancer J Clin 50:7–33
Schwartz GF et al (2000) Consensus Conference on the treatment of In Situ Ductal Carcinoma of the Breast April 22–25, 1999. Cancer 88:946–954
Silverstein MJ et al (1996) A prognostic index for DCIS of the breast. Cancer 77:2267–2274
Vicini FA et al (2000) Impact of young age on outcome in patients with DCIS treated with breast conserving therapy. J Clin Oncol 18:296–306
Goldstein NS et al (2000) Differences in the pathologic features of DCIS of the breast based on patient age. Cancer 88:2552–2260
Jhingran A et al (2002) Age as predictor of outcome for women with DCIS treated with breast conserving surgery and radiation: the University of Texas M.D. Anderson Cancer Center experience. Int J Rad Oncol 54(3):804–809
Silverstein MJ (2003) The University of Southern California/Van Nuys prognostic index for DCIS of the breast. Am J Surg 186:337–343
Silverstein MJ et al (1997) Use of predictors of recurrence to plan therapy for DCIS of the breast. Oncology (Huntingt) 11:393–410
Silverstein MJ (1997) Predicting local recurrences in patients with DCIS in Ductal Carcinoma of the Breast. Williams and Wilkins, Baltimore pp 271–283
Tavassoli FA et al (1992) Intraductal carcinoma in: anonymous pathology of the breast. Appleton and Lange, New York, pp 238–243
Holland PA et al (1998) The importance of complete excision in the prevention of local recurrence of DCIS. Br J Cancer 77:110–114
Boland GP et al (2003) Value of the Van Nuys prognostic index in prediction of recurrence of DCIS after breast conserving surgery. Br J Surg 90:426–432
Silverstein MJ et al (1996) Developing a prognostic index for DCIS of the breast. Are we there yet? Cancer 78:1138–1140
Silverstein MJ et al (1995) Prognostic classification of breast ductal carcinoma in situ. Lancet 345:1154–1157
Douglas-Jones AG et al (1996) A critical appraisal of six modern classifications of DCIS of the breast: correlation with grade of associated invasive carcinoma. Histopathology 29:397–409
De Mascarel I et al (2000) Application of the VNPI in a retrospective series of 367 DCIS of the breast examinated by serial macroscopic sectioning: practical considerations. Breast Cancer Res Treat 61:151–159
Solin LJ et al (2005) Long term outcome after breast conservation treatment with radiation for mammographically detected DCIS of the breast. Cancer 103(6):1137–1146
Rodrigues N et al (2002) Correlation of clinical and pathologic features with outcome in patients with DCIS of the breast treated with breast conserving surgery and radiotherapy. Int J Rad Oncol Biol Phys 54(5):1331–1335
Denoux Y et al (2001) Evaluation of predictive factors, particularly the VNPI, of local recurrence in DCIS of the breast: study of 166 cases with conservative treatment and review of literature. Bull Cancer 88(4):419–425
Fisher ER et al (1995) Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) protocol B-17. Cancer 75:1310–1319
Mc Cormick B et al (1991) DCIS of the breast: an analysis of local control after conservation surgery and radiotherapy. Int J Rad Oncol Biol Phys 231:289–292
Vicini FA et al (1997) DCIS detected in the mammographic era: an analysis of clinical, pathologic and treatment-related factors affecting outcome with breast conserving therapy. Int J Rad Oncol Biol Phys 39:627–635
Solin LJ et al (2001) Mammographically detected DCIS of the breast treated with breast conserving surgery and definitive breast irradiation: long term outcome and prognostic significance of patient age and margin status. Int J Rad Oncol Biol Phys 50:991–1002
Silverstein MJ et al (2003) DCIS: USC/Van Nuys Prognostic Index and the impact of margin status. Breast 12:457–471
Cutuli B et al (2002) Breast Conserving therapy for DCIS of the breast: the French cancer centers’ experience. Int J Rad Oncol Biol Phys 53(4):868–879
Kestin LL et al (2000) Factors associated with local recurrence of mammographically detected DCIS in patients given breast conserving therapy. Cancer 88:596–607
Lagios MD et al (1990) DCIS: pathology and treatment. Surg Clin North Am 70:853–871
Solin LJ et al (1994) Salvage treatment for local recurrence following breast conserving surgery and definitive irradiation for DCIS of the breast. Int J Rad Oncol Biol Phys 30:3–9
Schwartz G (1994) The role of excision and surveillance alone in subclinical DCIS of the breast. Oncology 8:21–26
Solin LJ et al (1996) Mammographically detected clinically occult DCIS treated with breast conserving surgery and definitive breast irradiation. Cancer J Sci Am 2:158–165
Nakamura S et al (2002) Breast Conserving therapy for DCIS: a 20 year experience with excision plus radiation therapy. Am J Surg 184:403–409
Van Zee KJ et al (1999) Long term follow up of women with DCIS treated with breast conserving surgery: the effect of age. Cancer 86:1757–1767
Fowble B et al (1997) Results of conservative surgery and radiation for mammographically detected DCIS. Int J Rad Oncol Biol Phys 38:949–957
Kuske RR et al (1993) Breast conservation therapy for intraductal carcinoma of the breast. Int J Rad Oncol Biol Phys 26:391–396
Mc Cormick B et al (1991) Radiation therapy in breast conservation patients and postmastectomy. Semin Surg Oncol 7:278–282
Kestin LL et al (2000) Mammographically detected DCIS treated with conservative surgery with or without radiation therapy: patterns of failure and 10 year results. Ann Surg 231:235–245
Fourquet A et al (1997) Ductal Carcinoma in Situ of the breast. In: Silverstein MJ (ed) Philadelphia Williams and Wilkins
Recht A et al (1985) Intraductal carcinoma of the breast: results of treatment with excisional biopsy and irradiation. J Clin Oncol 3:1339–1343
White J et al (1995) Outcome and prognostic factors for local recurrence in mammographically detected DCIS of the breast treated with conservative surgery and radiation therapy. Int J Rad Oncol Biol Phys 31:791–797
Bornstein BA et al (1991) Results of treating DCIS of the breast with conservative surgery and radiation therapy. Cancer 67:7–13
Vicini F, Recht A (2002) Age at diagnosis and outcome for women with Ductal Carcinoma in situ of the breast: a critical review of the literature. J Clin Oncol 20(11):2736–2744
Fisher B et al (1993) Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 328:1581–1586
Julien JP et al (2000) Radiotherapy in breast conserving treatment for DCIS: first results of the EORTC randomised phase III trial 10853 EORTC Breast Cancer Cooperative Group and Radiotherapy Group. Lancet 355:528–533
Bijker N et al (2001) Risk factors for recurrence and metastases after breast conserving therapy for DCIS: analysis of European Organization for Research and Treatment of Cancer trial 10853. J Clin Oncol 19:2263–2271
Houghton J et al (2003) Radiotherapy and tamoxifen in women with completely excised DCIS of the breast in the UK, Australia and New Zealand: randomised controlled trial. Lancet 362:95–102
Silverstein MJ (2002) The University of Southern California/Van Nuys prognostic index in ductal Carcinoma in situ of the breast, 2nd ed. Philadelphia Lippincott Wlliams and Wlinkins, pp 459−473
Fisher B et al (2001) Prevention of invasive breast cancer in women with DCIS: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol 28:200–218
Silverstein MJ et al (1999) The influence of margin width on local control in patients with DCIS of the breast. N Engl J Med 340:1455–1461
Wong JS et al (2006) Prospective study of wide excision alone for DCIS of the breast. J Clin Oncol 24(7):1031–1036
Cutuli B, Cohen Solal LeNir et al (2001) Ductal carcinoma in situ of the breast results of conservative and radical treatments in 716 patients. Eur J Cancer 37(18):2365–2372
Ciatto S, Bonardo R, Cataliotti R et al (1990) Intraductal breast carcinoma. Review of a multicenter series of 350 cases. Coordinating Center and Writing Committee of FONCAM (National Task Force for Breast Cancer), Italy Tumori. 31 Dec 1990; 76(6):552−554
Lagios M, Silverstein MJ (2001) Sentinel node biopsy for patients with DCIS: a dangerous and unwarranted direction. Ann Surg Oncol 8(4):275–277
Keshtgar MR et al (2002) Clinical role of sentinel lymph node biopsy in breast cancer. Lancet Oncol 3:105–110
Klauber-DeMore N et al (2000) Sentinel lymph node biopsy: is it indicated in patients with high risk DCIS and DCIS with microinvasion? Ann Surg Oncol 7:636–642
Silverstein MJ et al (2001) Predicting axillary nodal positivity in 2282 patients with breast carcinoma. World J Surg 25:767–772
Kelly T et al (2003) Axillary lymph node metastases in patients with a final diagnosis of DCIS. Am J Surg 186:368–370
Intra M et al (2003) Axillary sentinel lymph node biopsy in patients with pure DCIS of the breast. Arch Surg 138:309–313
Pendas S et al (2000) Sentinel node biopsy in DCIS patients. Ann Surg Oncol 7:15–20
Moore KH et al (2004) Immunohistochemically detected tumor cells in the sentinel lymph nodes of patients with breast carcinoma. Cancer 100(5):929–934
Lara J et al (2003) The relevance of occult axillary micrometastasis in DCIS. Cancer 98(10):2105–2113
Mittendorf MEA et al (2005) Core biopsy diagnosis of DCIS: an indication for sentinel node biopsy. Curr Surg 62(2):253–257
Weaver D (2003) Occult “micrometastases” in DCIS. Cancer 98(10):2083–2087
Fisher B et al (1999) Tamoxifen in treatment of intraductal breast cancer: NSABP project B-24 randomised controlled trial. Lancet 353:1993–2000
Hiramatsu H et al (1995) Local recurrence after conservative surgery and radiation therapy for DCIS. Cancer J Sci Am 1:55
Fisher B et al (1998) Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from NSABP project B-17. J Clin Oncol 16:441–452
Szelei-Stevens KA et al (2000) The influence of young age and positive family history of breast cancer on the prognosis of DCIS treated by excision with or without radiation therapy or by mastectomy. Int J Rad Oncol Biol Phys 48:943–949
Harris EE et al (2000) Relationship of family history and outcome after breast conservation therapy in women with DCIS of the breast. Int J Rad Oncol Biol Phys 48:933–941
Author information
Authors and Affiliations
Corresponding author
Additional information
Author’s contributions
Conception and Design: S. Di Saverio, M. Taffurelli, D. Santini
Provision of study materials or patients: M. Taffurelli, D. Santini, S. Mignani, S. Di Saverio
Collection and Assembly of Data: S. Di Saverio, T. Fogacci
Data Analysis and Interpretation: S. Di Saverio, M. Taffurelli, D. Santini, F. Catena, L. Ansaloni
Manuscript Writing: S. Di Saverio
Final Approval of Manuscript: S. Di Saverio, F. Catena, D. Santini, L. Ansaloni, T. Fogacci, S. Mignani, A. Leone, F. Gazzotti, S. Gagliardi, A. De Cataldis, M. Taffurelli
Rights and permissions
About this article
Cite this article
Di Saverio, S., Catena, F., Santini, D. et al. 259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: a retrospective review with long term follow up. Breast Cancer Res Treat 109, 405–416 (2008). https://doi.org/10.1007/s10549-007-9668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9668-7