Abstract
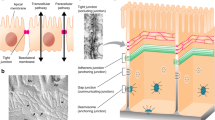
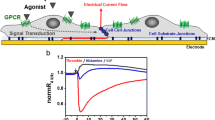
Silicon nanomembranes are ultrathin, highly permeable, optically transparent and biocompatible substrates for the construction of barrier tissue models. Trans-epithelial/endothelial electrical resistance (TEER) is often used as a non-invasive, sensitive and quantitative technique to assess barrier function. The current study characterizes the electrical behavior of devices featuring silicon nanomembranes to facilitate their application in TEER studies. In conventional practice with commercial systems, raw resistance values are multiplied by the area of the membrane supporting cell growth to normalize TEER measurements. We demonstrate that under most circumstances, this multiplication does not ‘normalize’ TEER values as is assumed, and that the assumption is worse if applied to nanomembrane chips with a limited active area. To compare the TEER values from nanomembrane devices to those obtained from conventional polymer track-etched (TE) membranes, we develop finite element models (FEM) of the electrical behavior of the two membrane systems. Using FEM and parallel cell-culture experiments on both types of membranes, we successfully model the evolution of resistance values during the growth of endothelial monolayers. Further, by exploring the relationship between the models we develop a ‘correction’ function, which when applied to nanomembrane TEER, maps to experiments on conventional TE membranes. In summary, our work advances the the utility of silicon nanomembranes as substrates for barrier tissue models by developing an interpretation of TEER values compatible with conventional systems.






Similar content being viewed by others
References
A. Acheampong, J.-L. Vincent, A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care. 19(1), 251 (2015)
A.A. Agrawal, B. Nehilla, K. Reisig, T. Gaborski, D. Fang, C. Striemer, P. Fauchet, J. McGrath, Porous nanocrystalline silicon membranes as highly permeable and molecularly thin substrates for cell culture. Biomaterials. 31(20), 5408–17 (2010)
A.D. ávan der Meer, H. JungáKim, M.W. ávan der Helm, A. den Berg, Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems. Lab Chip. 15(3), 745–52 (2015)
K. Benson, S. Cramer, H.-J. Galla, Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS. 10(1), 5 (2013)
S.N. Bhatia, D.E. Ingber, Microfluidic organs-on-chips. Nat. Biotechnol. 32(8), 760–72 (2014)
M. Bindschadler, J.L. McGrath, Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 120(5), 876–84 (2007)
M.I. Bogorad, J. DeStefano, J. Karlsson, A.D. Wong, S. Gerecht, P.C. Searson, in vitro microvessel models. Lab Chip. 15(22), 4242–55 (2015)
R. Booth, H. Kim, Characterization of a microfluidic in vitro model of the blood-brain barrier (μ bbb). Lab Chip. 12(10), 1784–92 (2012)
R. Booth, S. Noh, H. Kim, A multiple-channel, multiple-assay platform for characterization of full-range shear stress effects on vascular endothelial cells. Lab Chip. 14(11), 1880–90 (2014)
R.N. Carter, S.M. Casillo, A.R. Mazzocchi, J.-P.S. DesOrmeaux, J.A. Roussie, T.R. Gaborski, Ultrathin transparent membranes for cellular barrier and co-culture models. Biofabrication. 9(1), 015,019 (2017)
S.M. Casillo, A.P. Peredo, S.J. Perry, H.H. Chung, Gaborski T.R, Membrane pore spacing can modulate endothelial cell–substrate and cell–cell interactions. ACS Biomaterials Sci. Eng. (2017)
H.H. Chung, C.K. Chan, T.S. Khire, G.A. Marsh, A. Clark, R.E. Waugh, J.L. McGrath, Highly permeable silicon membranes for shear free chemotaxis and rapid cell labeling. Lab Chip. 14(14), 2456–68 (2014)
C. Crone, O. Christensen, Electrical resistance of a capillary endothelium. J. Gen. Physiol. 77(4), 349–71 (1981)
C. Crone, S. Olesen, Electrical resistance of brain microvascular endothelium. Brain Res. 241(1), 49–55 (1982)
L. Cucullo, M. Hossain, W. Tierney, D. Janigro, A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci. 14(1), 18 (2013)
J. DesOrmeaux, J. Winans, S. Wayson, T. Gaborski, T. Khire, C. Striemer, J. McGrath, Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates. Nanoscale. 6(18), 10,798–805 (2014)
N.J. Douville, Y.-C. Tung, R. Li, J.D. Wang, M.E. El-Sayed, S. Takayama, Fabrication of two-layered channel system with embedded electrodes to measure resistance across epithelial and endothelial barriers. Anal. Chem. 82(6), 2505 (2010)
R.S. Eisenberg, E.A. Johnson, Three-dimensional electrical field problems in physiology. Prog. Biophys. Molecul. Biol. 20, 1–65 (1970)
N. Ferrell, R.R. Desai, A.J. Fleischman, S. Roy, H.D. Humes, W.H. Fissell, A microfluidic bioreactor with integrated transepithelial electrical resistance (teer) measurement electrodes for evaluation of renal epithelial cells. Biotechnol. Bioeng. 107(4), 707–16 (2010)
O. Henry, R. Villenave, M. Cronce, W. Leineweber, M. Benz, D Ingber, Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (teer) measurements of human epithelial barrier function. Lab Chip (2017)
D. Huh, D.C. Leslie, B.D. Matthews, J.P. Fraser, S. Jurek, G.A. Hamilton, K.S. Thorneloe, M.A. McAlexander, D.E. Ingber, A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Translat. Med. 4(159), 159ra147–159ra147 (2012)
R. Ishimatsu, J. Kim, P. Jing, C.C. Striemer, D.Z. Fang, P.M. Fauchet, J.L. McGrath, S. Amemiya, Ion-selective permeability of an ultrathin nanoporous silicon membrane as probed by scanning electrochemical microscopy using micropipet-supported ities tips. Anal. Chem. 82(17), 7127–34 (2010)
E.A. Jaffe, Cell biology of endothelial cells. Human Pathol. 18(3), 234–9 (1987)
D.G. Johnson, T.S. Khire, Y.L. Lyubarskaya, K.J. Smith, J.-P.S. DesOrmeaux, J.G. Taylor, T.R. Gaborski, A.A. Shestopalov, C.C. Striemer, J.L. McGrath, Ultrathin silicon membranes for wearable dialysis. Adv. Chronic Kidney Dis. 20(6), 508–15 (2013)
L.C. Kelley, L.L. Lohmer, E.J. Hagedorn, D.R. Sherwood, Traversing the basement membrane in vivo: A diversity of strategies. J. Cell. Biol. 204(3), 291–302 (2014)
H.J. Kim, D. Huh, G. Hamilton, D.E. Ingber, Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 12(12), 2165–74 (2012)
W.L. Lee, A.S. Slutsky, Sepsis and endothelial permeability. England J. Med. 363(7), 689 (2010)
A.R. Mazzocchi, A.J. Man, J.-P.S. DesOrmeaux, T.R. Gaborski, Porous membranes promote endothelial differentiation of adipose-derived stem cells and perivascular interactions. Cell. Mol. Bioeng. 7(3), 369–78 (2014)
B.J. Nehilla, N. Nataraj, T.R. Gaborski, J.L. McGrath, Endothelial vacuolization induced by highly permeable silicon membranes. Acta Biomaterialia. 10(11), 4670–7 (2014)
M.A.L. Pinheiro, G. Kooij, M.R. Mizee, A. Kamermans, G. Enzmann, R. Lyck, M. Schwaninger, B. Engelhardt, H.E. de Vries, Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochimica et Biophysica Acta (BBA)-Molecular Basis Disease. 1862(3), 461–71 (2016)
S. Ryu, J. Yoo, Y. Jang, J. Han, S.J. Yu, J. Park, S.Y. Jung, K.H. Ahn, S.G. Im, K. Char, Nanothin coculture membranes with tunable pore architecture and thermoresponsive functionality for transfer-printable stem cell-derived cardiac sheets. ACS Nano. 9(10), 10,186–202 (2015)
C.M. Sakolish, M.B. Esch, J.J. Hickman, M.L. Shuler, G.J. Mahler, Modeling barrier tissues in vitro: Methods, achievements, and challenges. EBioMedicine. 5, 30–9 (2016)
J. Seok, H.S. Warren, A.G. Cuenca, M.N. Mindrinos, H.V. Baker, W. Xu, D.R. Richards, G.P. McDonald-Smith, H. Gao, L. Hennessy, Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. 110(9), 3507–12 (2013)
J. Snyder, A. Clark, D. Fang, T. Gaborski, C. Striemer, P. Fauchet, J. McGrath, An experimental and theoretical analysis of molecular separations by diffusion through ultrathin nanoporous membranes. J. Membrane Sci. 369(1), 119–29 (2011)
J.L. Snyder, J. Getpreecharsawas, D.Z. Fang, T.R. Gaborski, C.C. Striemer, P.M. Fauchet, D.A. Borkholder, J.L. McGrath, High-performance, low-voltage electroosmotic pumps with molecularly thin silicon nanomembranes. Proc. Nat. Acad. Sci. 110(46), 18,425–30 (2013)
B. Srinivasan, A.R. Kolli, M.B. Esch, H.E. Abaci, M.L. Shuler, J.J. Hickman, Teer measurement techniques for in vitro barrier model systems. J. Laborat. Autom. 20(2), 107–26 (2015)
C.C. Striemer, T.R. Gaborski, J.L. McGrath, P.M. Fauchet, Charge-and size-based separation of macromolecules using ultrathin silicon membranes. Nature. 445(7129), 749–53 (2007)
T. Sun, E.J. Swindle, J.E. Collins, J.A. Holloway, D.E. Davies, H. Morgan, On-chip epithelial barrier function assays using electrical impedance spectroscopy. Lab Chip. 10(12), 1611–7 (2010)
M.L. Sutherland, K.M. Fabre, D.A. Tagle, The national institutes of health microphysiological systems program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res. Therapy. 4(1), I1 (2013). https://doi.org/10.1186/scrt361
N. Tandon, A. Marsano, R. Maidhof, K. Numata, C. Montouri-Sorrentino, C. Cannizzaro, J. Voldman, G. Vunjak-Novakovic, Surface-patterned electrode bioreactor for electrical stimulation. Lab Chip. 10 (6), 692–700 (2010)
K. Tanner, Regulation of the basement membrane by epithelia generated forces. Phys. Biol. 9(6), 065,003 (2012)
P.A. Vogel, S.T. Halpin, R.S. Martin, D.M. Spence, Microfluidic transendothelial electrical resistance measurement device that enables blood flow and postgrowth experiments. Anal. Chem. 83(11), 4296–301 (2011)
F.R. Walter, S. Valkai, A. Kincses, A. Petneházi, T. Czeller, S. Veszelka, P. Ormos, M.A. Deli, A. Dér, A versatile lab-on-a-chip tool for modeling biological barriers. Sens. Actuators B. 222, 1209–19 (2016)
Y.I. Wang, H.E. Abaci, M.L. Shuler, Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 114(1), 184–94 (2017)
I. Woolhouse, D. Bayley, P. Lalor, D. Adams, R. Stockley, Endothelial interactions of neutrophils under flow in chronic obstructive pulmonary disease. Europ. Respir. J. 25(4), 612–7 (2005)
J. Yeste, X. Illa, A. Guimerà, R. Villa, A novel strategy to monitor microfluidic in-vitro blood-brain barrier models using impedance spectroscopy. In: SPIE Microtechnologies, International Society for Optics and Photonics, pp. 95,180N–95,180N (2015)
Acknowledgements
Authors would like to thank Dr. Henry Chung, Zachery Hulings and Tucker Bergin for their help in COMSOL modeling, and Thomas Andolsek in obtaining the TEER data for validation experiments. They also acknowledge the support of Dr. Allison Elder (Department of Environmental Medicine, University of Rochester) during the early days of this project. This work was supported by funding from the National Institutes of Health under program project grant number: 5 R01 HL125265.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funds supporting this research were provided by the US Public Health Service under NIH grant number 5R01 HL125265
Tejas S. Khire and Barrett J. Nehilla contributed equally to this paper
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khire, T.S., Nehilla, B.J., Getpreecharsawas, J. et al. Finite element modeling to analyze TEER values across silicon nanomembranes. Biomed Microdevices 20, 11 (2018). https://doi.org/10.1007/s10544-017-0251-7
Published:
DOI: https://doi.org/10.1007/s10544-017-0251-7