Abstract
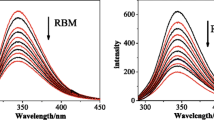
Ruthenium compounds are highly regarded as metallo-drug candidates. Many studies have focused their attention on the interaction between ruthenium complexes with their possible biological targets. The interaction of ruthenium complexes with transport proteins, enzymes and peptides is of great importance for understanding their biodistribution and mechanism of action, therefore, the development of an anti-cancer therapy involving ruthenium complexes has recently shifted from DNA targeting towards protein targeting. With the aim of gaining insight into possible interactions between ruthenium complexes with biologically relevant proteins, we have studied the interaction of cis-dichlorobis(2,2′-bipyridyl-4,4′-dicarboxylic acid)ruthenium(II) complex [Ru(II)(dcbpy)2Cl2], which previously showed good potency in photo-dynamic chemotherapy, with bovine serum albumin (BSA), phospholipase A2 (PLA2) and glutathione (GSH). Binding constants and possible number of binding sites to mentioned proteins and peptide are investigated by ultraviolet–visible spectroscopy and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry (MALDI TOF MS). The complex binding affinities were in the following order: PLA2 > BSA > GSH. Moreover, genotoxic profile of the complex, tested on peripheral blood lymphocytes as a model system, was also promising.






Similar content being viewed by others
References
Allardyce C, Dyson P (2001) Ruthenium in medicine: current clinical uses and future prospects. Plat Metal Rev 45:62–69
Anthonysamy A, Lee Y, Karunagaran B et al (2011) Molecular design and synthesis of ruthenium(II) sensitizers for highly efficient dye-sensitized solar cells. J Mater Chem 21:12389–12397. doi:10.1039/C1JM11760B
Antonarakis ES, Emadi A (2010) Ruthenium-based chemotherapeutics: are they ready for prime time? Cancer Chemother Pharmacol 66:1–9. doi:10.1007/s00280-010-1293-1
Aoki J (2004) Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol 15:477–489. doi:10.1016/j.semcdb.2004.05.001
Barton J (1994) Metal/nucleic-acid interactions. In: Bertini I, Gray H, Lippard S, Valentine JS (eds) Bioinorganic chemistry, University Science Books, Mill Valley, California, pp 455–500
Bertini I (1994) Bioinorganic chemistry. Mill Valley
Bertolini J, Goss N, Curling J (2012) Production of plasma proteins for therapeutic use. Wiley, London
Callegher E, Seraglia R, Vettore M et al (2009) Kinetics of albumin homocysteinylation measured with matrix-assisted laser/desorption ionization mass spectrometry versus with a radioactive tracer. Rapid Commun Mass Spectrom 23:3837–3842. doi:10.1002/rcm.4290
Calvert P, Yao K-S, Hamilton TC, O’Dwyer PJ (1998) Clinical studies of reversal of drug resistance based on glutathione. Chem Biol Interact 111–112:213–224. doi:10.1016/S0009-2797(98)00008-8
Casini A, Gabbiani C, Michelucci E et al (2009) Exploring metallodrug-protein interactions by mass spectrometry: comparisons between platinum coordination complexes and an organometallic ruthenium compound. J Biol Inorg Chem 14:761–770. doi:10.1007/s00775-009-0489-5
Cini R, Tamasi G, Defazio S et al (2003) Study of ruthenium(II) complexes with anticancer drugs as ligands. Design of metal-based phototherapeutic agents. Inorg Chem 42:8038–8052. doi:10.1021/ic0349095
Cuendet M, Pezzuto JM (2000) The role of cyclooxygenase and lipoxygenase in cancer chemoprevention. Drug Metabol Drug Interact 17:109–157
Cummings BS, McHowat J, Schnellmann RG (2000) Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther 294:793–799
da Paula Silva MM, Pich CT, Petronilho F et al (2005) Antioxidant activity of new ruthenium compounds. Redox Rep Commun Free Radic Res 10:139–143. doi:10.1179/135100005X38897
Das D, Dutta A, Mondal P (2014) Interactions of the aquated forms of ruthenium(III) anticancer drugs with protein: a detailed molecular docking and QM/MM investigation. RSC Adv 4:60548–60556. doi:10.1039/C4RA10630J
Domínguez CSH, Hernández P (2013) Use of cucurbit [6] uril as a modifier in the electrochemical determination of antitumor platinum (II) complex: trans-[PtCl2(dimethylamine) (isopropylamine)]. Application to biological samples. Am J Anal Chem 04:314–322. doi:10.4236/ajac.2013.46040
Fenech M (1993) The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
Ghuman J, Zunszain PA, Petitpas I et al (2005) Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353:38–52. doi:10.1016/j.jmb.2005.07.075
Godwin AK, Meister A, O’Dwyer PJ et al (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA 89:3070–3074
Hartinger CG, Zorbas-Seifried S, Jakupec MA et al (2006) From bench to bedside–preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). J Inorg Biochem 100:891–904. doi:10.1016/j.jinorgbio.2006.02.013
Hartinger CG, Jakupec MA, Zorbas-Seifried S et al (2008) KP1019, a new redox-active anticancer agent–preclinical development and results of a clinical phase I study in tumor patients. Chem Biodivers 5:2140–2155. doi:10.1002/cbdv.200890195
Ibrahim MS, Shehatta IS, Al-Nayeli AA (2002) Voltammetric studies of the interaction of lumazine with cyclodextrins and DNA. J Pharm Biomed Anal 28:217–225. doi:10.1016/S0731-7085(01)00624-0
Jiang C-W, Chao H, Hong X-L et al (2003) Enantiopreferential DNA-binding of a novel dinuclear complex [(bpy)2Ru(bdptb)Ru(bpy)2]4+. Inorg Chem Commun 6:773–775. doi:10.1016/S1387-7003(03)00109-6
Kamčeva T, Flemmig J, Damnjanović B et al (2011) Inhibitory effect of platinum and ruthenium bipyridyl complexes on porcine pancreatic phospholipase A2. Met Integr Biometal Sci 3:1056–1063. doi:10.1039/c1mt00088h
Kamčeva T, Radisavljević M, Vukićević I et al (2013) Interactions of platinum and ruthenium coordination complexes with pancreatic phospholipase A2 and phospholipids investigated by MALDI TOF mass spectrometry. Chem Biodivers 10:1972–1986. doi:10.1002/cbdv.201300141
Karki S, Ravikumar TK, Khatiyar A et al (2014) Synthesis, cytostatic and antiviral activity of some ruthenium (II) complexes. Turk J Pharm Sci 11:163–175
Kelley SL, Basu A, Teicher BA et al (1988) Overexpression of metallothionein confers resistance to anticancer drugs. Science 241:1813–1815
Kostova I (2006) Ruthenium complexes as anticancer agents. Curr Med Chem 13:1085–1107
Kragh-Hansen U (1981) Molecular aspects of ligand binding to serum albumin. Pharmacol Rev 33:17–53
Kratz F, Senter P, Steinhagen H (2013) Drug delivery in oncology: from basic research to cancer therapy, 3 volume set. Wiley, London
Krause-Heuer AM, Price WS, Aldrich-Wright JR (2012) Spectroscopic investigations on the interactions of potent platinum(II) anticancer agents with bovine serum albumin. J Chem Biol 5:105–113. doi:10.1007/s12154-012-0074-1
Laye JP, Gill JH (2003) Phospholipase A2 expression in tumours: a target for therapeutic intervention? Drug Discov Today 8:710–716
Li X-Y, Li T-H, Guo J-S et al (2012) PEGylation of bovine serum albumin using click chemistry for the application as drug carriers. Biotechnol Prog 28:856–861. doi:10.1002/btpr.1526
Li F, Collins JG, Keene FR (2015) Ruthenium complexes as antimicrobial agents. Chem Soc Rev 44:2529–2542. doi:10.1039/C4CS00343H
Lima AP, Pereira FC, Almeida MAP et al (2014) Cytoxicity and apoptotic mechanism of ruthenium(II) amino acid complexes in sarcoma-180 tumor cells. PLoS One 9:e105865. doi:10.1371/journal.pone.0105865
Liu JG, Ye BH, Zhang QL et al (2000) Enantiomeric ruthenium(II) complexes binding to DNA: binding modes and enantioselectivity. J Biol Inorg Chem JBIC Publ Soc Biol Inorg Chem 5:119–128
Liu Y-J, Zeng C-H, Yao J-H et al (2010) Synthesis, structure, DNA-binding properties, and cytotoxicity of ruthenium(II) polypyridyl complexes. Chem Biodivers 7:1770–1783. doi:10.1002/cbdv.200900213
Liu Y-J, Liang Z-H, Li Z-Z et al (2011) Ruthenium(II) polypyridyl complexes: synthesis and studies of DNA binding, photocleavage, cytotoxicity, apoptosis, cellular uptake, and antioxidant activity. DNA Cell Biol 30:829–838. doi:10.1089/dna.2010.1170
Luzuriaga L, Cerdá MF (2012) Analysis of the interaction between [Ru(phenanthroline)3]2+ and bovine serum albumin. Adv Biol Chem 02:262–267. doi:10.4236/abc.2012.23033
Magrioti V, Kokotos G (2010) Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin Ther Pat 20:1–18. doi:10.1517/13543770903463905
Messori L, Kratz F, Alessio E (1996) The interaction of the antitumor complexes Na[trans-RuCl4 (DMSO)(Im)] and Na[trans-RuCl4(DMSO)(Ind)] with apotransferrin: a spectroscopic study. Met-Based Drugs 3:1–9. doi:10.1155/MBD.1996.1
Messori L, Casini A, Vullo D et al (2000a) Effects of two representative antitumor ruthenium(III) complexes on thermal denaturation profiles of DNA. Inorganica Chim Acta 303:283–286. doi:10.1016/S0020-1693(99)00528-9
Messori L, Orioli P, Vullo D et al (2000b) A spectroscopic study of the reaction of NAMI, a novel ruthenium(III)anti-neoplastic complex, with bovine serum albumin. Eur J Biochem FEBS 267:1206–1213
Messori L, Vilchez FG, Vilaplana R et al (2000c) Binding of antitumor ruthenium(III) complexes to plasma proteins. Met-Based Drugs 7:335–342. doi:10.1155/MBD.2000.335
Meyer MC, Rastogi P, Beckett CS, McHowat J (2005) Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des 11:1301–1312
Morinaga O, Shoyama Y (2012) Quality control of Rheum and Cassia species by immunological methods using monoclonal antibodies against sennosides. In: Akyar I (ed) Latest research into quality control. InTech. doi:10.5772/51272
Motswainyana WM, Ajibade PA (2015) Anticancer activities of mononuclear ruthenium(II) coordination complexes. Adv Chem 2015:e859730. doi:10.1155/2015/859730
Motswainyana WM, Ajibade PA, Motswainyana WM, Ajibade PA (2015) Anticancer activities of mononuclear ruthenium(II) coordination complexes, anticancer activities of mononuclear ruthenium(II) coordination complexes. Adv Chem Adv Chem 2015:e859730. doi:10.1155/2015/859730
Nakanishi M, Rosenberg DW (2006) Roles of cPLA2α and arachidonic acid in cancer. Biochim Biophys Acta 1761:1335–1343. doi:10.1016/j.bbalip.2006.09.005
Nazeeruddin MK, Kay A, Rodicio I et al (1993) Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X=Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc 115:6382–6390. doi:10.1021/ja00067a063
Nazeeruddin MK, Zakeeruddin SM, Humphry-Baker R et al (1999) Acid-base equilibria of (2,2′-bipyridyl-4,4′-dicarboxylic acid)ruthenium(II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. Inorg Chem 38:6298–6305
Nešić M, Popović I, Leskovac A et al (2016) Testing the photo-sensitive nanocomposite system for potential controlled metallo-drug delivery. Opt Quantum Electron 48:1–7. doi:10.1007/s11082-016-0421-5
Odenheimer B, Wolf W (1982) Reactions of cisplatin with sulfur-containing amino acids and peptides I. Cysteine and glutathione. Inorg Chim Acta 66:L41–L43. doi:10.1016/S0020-1693(00)85769-2
Radisavljević M, Kamčeva T, Bugarčić ŽD, Petković M (2013) Inhibitory effect of cisplatin and [Pt(dach)Cl2] on the activity of phospholipase A2. J Enzyme Inhib Med Chem 28:651–660. doi:10.3109/14756366.2012.666539
Sathiya Kamatchi T, Chitrapriya N, Kim SK et al (2013) Influence of carboxylic acid functionalities in ruthenium (II) polypyridyl complexes on DNA binding, cytotoxicity and antioxidant activity: synthesis, structure and in vitro anticancer activity. Eur J Med Chem 59:253–264. doi:10.1016/j.ejmech.2012.11.024
Sava G, Pacor S, Bergamo A et al (1995) Effects of ruthenium complexes on experimental tumors: irrelevance of cytotoxicity for metastasis inhibition. Chem Biol Interact 95:109–126
Sava G, Alessio E, Bergamo A, Mestroni G (1999) Sulfoxide ruthenium complexes: non-toxic tools for the selective treatment of solid tumour metastases. In: Clarke PMJ, Sadler PPJ (eds) Metallopharmaceuticals I. Springer, Berlin, pp 143–169
Shah A, Khan AM, Usman M et al (2009) Thermodynamic characterization of dexamethasone sodium phosphate and its complex with DNA as studied by conductometric and spectroscopic techniques. J Chil Chem Soc 54:134–137. doi:10.4067/S0717-97072009000200007
Soebbing S (2008) Incorporation of histidine-rich metal-binding sites onto small protein scaffolds: implications for imaging, therapeutics, and catalysis. ProQuest
Sunder Anchuri S, Thota S, Yerra R, Dhulipala S (2012) In vitro antioxidant activity of some novel synthetic mononuclear ruthenium (II) compounds. Lett Drug Des Discov 9:421–425. doi:10.2174/157018012799859927
Traverso N, Ricciarelli R, Nitti M et al (2013) Role of glutathione in cancer progression and chemoresistance, role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013:e972913. doi:10.1155/2013/972913
Velders AH, Pazderski L, Ugozzoli F et al (1998) Synthesis, characterization and crystal structure of trans-aquatrichlorobis (5,7-dimethyl[1,2,4]triazolo[1,5-a]pyrimidine-N-3)ruthenium(III) monohydrate. Inorg Chim Acta 273:259–265
Wang And X, Guo Z (2007) The role of sulfur in platinum anticancer chemotherapy. Anticancer Agents Med Chem 7:19–34
Xu L, Zhong N-J, Xie Y-Y et al (2014) Synthesis, characterization, in vitro cytotoxicity, and apoptosis-inducing properties of ruthenium(II) complexes. PLoS One 9:e96082. doi:10.1371/journal.pone.0096082
Yamashita S, Ogawa M, Sakamoto K et al (1994) Elevation of serum group II phospholipase A2 levels in patients with advanced cancer. Clin Chim Acta Int J Clin Chem 228:91–99
Acknowledgments
This work was supported by the Serbian Ministry of Education, Science and Technological Development, Grant Nos. 172011 and 172023.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nešić, M., Popović, I., Leskovac, A. et al. Biological activity and binding properties of [Ru(II)(dcbpy)2Cl2] complex to bovine serum albumin, phospholipase A2 and glutathione. Biometals 29, 921–933 (2016). https://doi.org/10.1007/s10534-016-9964-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9964-y