Abstract
Invasive mice (Mus spp.) can negatively impact island species and ecosystems. Because fewer island rodent eradications have been attempted for mice compared to rats (Rattus spp.), less is known about efficacy and palatability of rodenticide baits for mouse eradications. We performed a series of bait acceptance and efficacy cage trials using a standard formulation of brodifacoum-based rodenticide on wild-caught mice from Sand Island, Midway Atoll, to help inform a proposed eradication there. Mice were offered ad libitum brodifacoum pellets along with various alternative food sources, and a “no choice” treatment group received only bait pellets. Mortality in the no choice trial was 100%; however, when offered alternative foods, mice preferred the alternative diets to the bait, leading to low mortality (40%). Because there was concern that the bittering agent Bitrex® in the formulation may have reduced palatability, we conducted a subsequent trial comparing brodifacoum bait with and without Bitrex. Mortality in the with-Bitrex treatment group was slightly higher, indicating that the bittering agent was not likely responsible for low efficacy. Laboratory trials cannot account for the numerous environmental and behavioral factors that influence bait acceptance nor replicate the true availability of alternative food sources in the environment, so low efficacy results from these trials should be interpreted cautiously and not necessarily as a measure of the likelihood of success or failure of a proposed eradication.
Similar content being viewed by others
Introduction
Recent documentation of introduced house mice (Mus musculus) depredating nesting Laysan albatrosses (Phoebastria immutabilis) and black-footed albatrosses (P. nigripes) on Midway Atoll National Wildlife Refuge (MANWR; Duhr et al. 2019) highlights the potential threat these invasive rodents pose on insular species and ecosystems. While the negative impacts of invasive rats (Rattus spp.) to island ecosystems are well known (Harper and Bunbury 2015; Harris 2009; Towns et al. 2006; Varnham 2010) there is growing evidence that mice can be equally destructive, not just to seabird populations (Cuthbert and Hilton 2004; Wanless et al. 2007), but to all insular flora and fauna, as well as insular ecosystems themselves (Angel et al. 2009). Where mice are the only introduced mammals, such as at MANWR, their impacts can be severe, including the only examples of direct predation on adult seabirds (Angel et al. 2009).
In response to these negative impacts, various techniques have been developed to eradicate or control rodents to restore island systems. Today the primary rodent eradication method used to restore island systems of any size or with steep topography relies on aerial application of cereal-based bait pellets containing various second-generation anticoagulant rodenticides (Holmes et al. 2015; MacKay et al. 2007). Despite many documented successful rat eradications (Howald et al. 2007; Veitch et al. 2019, 2011) and associated positive conservation outcomes (Brooke et al. 2018; Croll et al. 2005; Jones et al. 2016), far fewer mouse eradications have been attempted than rat eradications (Howald et al. 2007). This disparity in eradication attempts between rats and mice means that, in general, more is known about the bait preferences and best practices for eradicating rats than for mice.
Mice are naturally more tolerant of anticoagulants, toxicity can vary depending on the population being studied and laboratory procedures (Wheeler et al. 2019), and genetic resistance can occur where these compounds have been used historically for controlling populations (Bailey and Eason 2000; Buckle and Prescott 2012; Pelz et al. 2005). Moreover, individual susceptibility to rodenticides can also vary considerably within and among populations (Cuthbert et al. 2011; O'Connor and Booth 2001; Wheeler et al. 2019). Therefore, comparable information is needed on susceptibility and palatability when establishing feasibility for a successful mouse eradication. This is a particular concern for the proposed eradication on MANWR, where anticoagulants have historically been used to control rodent populations. Thus, it is important to confirm that the proposed bait for the mouse eradication on MANWR is effective and palatable to wild-caught mice because their free-ranging counterparts are likely to have alternative food sources during an eradication operation.
Background
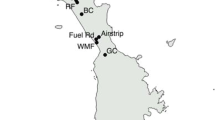
MANWR is located in the central North Pacific Ocean approximately 1850 km northwest of Honolulu (28°12′N 177°21′W) and is administered by the U.S. Fish and Wildlife Service (USFWS). Comprised of three low-lying coralline islands (Sand, Eastern, and Spit Islands) with a total land mass of approximately 6.2 km2, the refuge is an important breeding site for millions of seabirds, including the largest Laysan albatross breeding colony on the planet, and serves as a refuge for the critically endangered monk seal (Monachus schauinslandi) and Laysan duck (Anas laysanensis). A brodifacoum-based rodenticide was used to eradicate invasive black rats (Rattus rattus) from the atoll in 1996 (DIISE 2018), but invasive house mice are still present on Sand Island, the largest of the three islands (Fig. 1).

reproduced from Reynolds et al. (2012)
Map of Hawaiian archipelago and Midway Atoll comprised of Sand, Spit, and Eastern Islands. Figure
The USFWS has proposed to eradicate house mice from Sand Island using Brodifacoum-25D Conservation (B-25D; 0.0025% brodifacoum). B-25D is a restricted use pesticide registered with the U. S. Environmental Protections Agency (EPA Reg. No. 56228-37) by the U. S. Department of Agriculture Animal and Plant Health Inspection Service (USDA APHIS) and manufactured for USDA APHIS by Bell Laboratories (Madison, WI). In preparation, field studies to determine bait uptake rates and consumption by mice on Sand Island were conducted using a non-toxic formulation of the bait. This formulation included a fluorescing biomarker, pyranine, to confirm consumption of bait by mice and non-target species. In some trials, mice exhibited lower rates of pyranine detection than was expected (Island Conservation 2017, 2018), and one possible explanation was that mice were choosing alternative food items instead of the bait. Another concern raised by the field trials was that the non-toxic formulation used in those trials did not contain denatonium benzoate (trade name Bitrex®), a bittering agent that is typically added to deter consumption of bait by children and pets. This difference raised questions about the ability to extrapolate the results of the field trials into predictions for how mice were likely to interact with toxic bait containing Bitrex during an actual eradication attempt.
Here we report the results of bait palatability and efficacy trials to inform operational planning of the proposed eradication of mice from Sand Island. The objectives of the first part of this study were: (1) to evaluate the laboratory efficacy (percent mouse mortality) of B-25D bait pellets when offered alone or with alternative food sources with varying levels of palatability; and (2) to evaluate whether the biomarker pyranine affects palatability of the bait. Subsequent to ambiguous results from Part 1 of this study, we conducted Part 2 to: (1) evaluate methodological effects (individual versus group housing of mice); and (2) assess effects of the bittering agent Bitrex on palatability and efficacy.
Methods
Part 1: house mouse brodifacoum 25D efficacy and palatability trial
Study animals, housing, and general animal health monitoring
We captured a total of 123 wild mice using Trapper® 24/7™ traps (Bell Laboratories, Madison WI) from various sites on Sand Island representing a range of habitat types including coastal shrubs, bunch grass restoration sites, forests of Casuarina sp., mixed woodlands/buildings, non-native grasses and forbs, and mix/transition areas between habitats, during 6–8 September 2018. Following capture, all mice were transported to refuge headquarters and placed into large tubs for approximately 20 min and dusted with Drione® (1.0% pyrethrin) for control of ectoparasites. Groups of 4–6 mice were temporarily housed in 26 × 47.5 × 15 cm solid-bottom plastic shoebox cages and maintained with ad libitum access to maintenance diet feed pellets (Laboratory Rodent Diet 5001®, LabDiet, St. Louis, MO) and water, in a climate-controlled room (range: 72–79 °F) with a 12 h:12 h light:dark cycle. Each cage was lined with bedding, shredded kraft paper, and a small PVC tube (12 × 45 cm) for a refugium/shelter.
On September 11, 2018, mice from different capture locations were weighed and were assigned as randomly as possible among cages to minimize potential bias of capture location on bait palatability. Based on stratified body weights, two individuals of each sex were drawn from the available pool of mice and placed in group housing cages so that there were representative weight class mice in each cage and conditions would more accurately reflect behavior of wild mice on Midway. Daily health checks were conducted three times per day (~ 9 am, ~ 3 pm, ~ 9 pm) throughout all phases of both Part 1 and Part 2 of the study. Daily consumption rates were not evaluated to minimize disturbance and unnecessary handling of mice.
Acclimation
Mice were acclimated to holding conditions for five days pre-test with ad libitum access to LabDiet 5001 maintenance pellets and water. Any group housed mice exhibiting cage anxiety or aggressive behavior were removed and euthanized and replaced with spare mice from the same pool of quarantined animals. All mice were reweighed at the end of the five-day acclimation period and transferred to clean cages with fresh materials and water bottles and assigned to a treatment group.
Treatment groups
Five cages of four mice each (n = 20) were randomly assigned to each of five treatment groups (Table 1) as follows:
-
(1)
Control: the untreated control group was offered only ad libitum access to the standard EPA challenge diet, consisting of 65% cornmeal, 25% rolled oats, 5% sugar, and 5% corn oil by weight as per the EPA Pesticide Assessment Guidelines (Schneider 1982).
-
(2)
No-choice: this test group received ad libitum access to B-25D pellets only; the no-choice test is intended to assess efficacy of B-25D in the absence of alternative food sources.
-
(3)
Two-choice low-palatability alternative: in addition to B-25D pellets, this group received ad libitum access to the EPA challenge diet which was intended to represent a low-palatability alternative to the bait pellets.
-
(4)
Two-choice high-palatability alternative: in addition to B-25D pellets, this group received ad libitum access to a “mixed” diet of items presumed to be preferred food sources for mice, a mixture of local grass seeds (Eragrostis variabilis, Eleusine indica, and Cyperus polystachyos), Kaytee Fiesta Mouse and Rat food (Central Garden & Pet Company, Chilton, Wisconsin), Zilla Reptile Munchies Mealworm (dried mealworms; Central Garden & Pet Company, Chilton, Wisconsin), and Flucker’s® Freeze-dried Crickets (Flucker Farm, Port Allen, Louisiana) to ensure full nutrient availability. This test group was intended to represent a worst-case scenario for preferable alternative food availability during an eradication.
-
(5)
Two-choice placebo preference: previous field bait uptake trials utilized a non-toxic B-25D formulation that contained the biomarker pyranine. Because of concerns that pyranine may cause reduced palatability, this test group was offered a non-toxic version of B-25D containing pyranine for comparison of consumption results to toxic B-25D without pyranine. In addition to ad libitum access to non-toxic B-25D pellets, this test group also had access to the EPA challenge diet.
Mice were housed in groups for this part of the study, due to the large number of treatment groups, a combination of logistical and manpower constraints, and to increase the number of mice per treatment.
Bait exposure phase (4 days)
Mice were offered free choice ad libitum exposure to the test and respective challenge diets (Table 1) for four days, emulating the critical period of bait availability for all rodents following a single aerial bait application during eradication operations (Broome et al. 2017).
Forty grams of B-25D pellets (or non-toxic formulation) were scattered on the cage floor for all treatment groups except the control group. Because the alternative diets (i.e., EPA challenge diet or mixed diet) were not in solid pellets, 40 g were offered in two separate PVC cups. Test foods that were depleted were replenished (amount recorded) after two days so that all mice had ad libitum access to their respective treatment diets throughout the four-day exposure phase. Diets exposed to ambient humidity in the test room were expected to gain or lose small amounts of moisture from the air; therefore, three separate samples of each diet type were weighed and prepared in similar quantities and exposed to ambient room temperature/moisture. Changes in their weights were used to generate correction factors for consumption estimates during the trial.
At the end of the four-day exposure phase, all uneaten or spilled diets were removed and separated from any nesting or bedding material and fecal pellets. Diets were air-dried for 12–24 h then weighed and recorded to calculate consumption. Because individual mice were not marked, consumption estimates were calculated for each cage and averaged for each treatment. Palatability was calculated as the ratio of the mass of the bait consumed in a cage divided by the total mass of all of the food consumed in that cage (bait + alternate diet; O'Connor and Booth 2001). If only bait was eaten the palatability ratio would equal 1.0; a ratio of 0.0 would indicate that no bait was consumed.
Post-exposure monitoring (10 days)
At the end of the four-day bait exposure period, all mice were transferred to new cages with fresh materials and water bottles. All diets were replaced with approximately 40 g of maintenance feed pellets during the subsequent 10-day post-exposure phase, with feed replenished as needed. During daily health checks, cages were “spot cleaned” with areas of excessively soiled bedding removed and replaced with fresh bedding. Observations of mice found to exhibit symptoms of rodenticide poisoning were recorded.
Part 2: Bitrex palatability trial
Unexpectedly low mortality in the B-25D two-choice trials (Part 1) led to concern that the bittering agent denatonium benzoate (Bitrex®), may have negatively affected the palatability of the bait and prompted questions about the potential methodological artefacts of group housing of test mice [e.g., aggressive behavior(Forestier et al. 2018), social transmission of food preferences (Valsecchi and Galef 1989)]. This follow-up study evaluated the possible effects of Bitrex on the palatability of B-25D using individually housed wild-caught mice from Sand Island. Additionally, it was questioned whether a ten-day post-exposure monitoring period was adequate to ensure that all lethally intoxicated mice would expire before the end of the study, so the post-exposure monitoring was extended to twenty days.
Sixty-four additional wild-caught mice were captured on Sand Island from 10–11 January 2019. Except for housing individual mice in their own cage and the twenty-day post-exposure observational period, all testing protocols and animal care were the same as described in Part 1. Treatment groups were offered: (1) B-25D with Bitrex vs. EPA challenge diet, (2) B-25D without Bitrex vs. EPA challenge diet and (3) EPA challenge diet only (control).
Chemical analyses
For each part of this study, samples of toxic B-25D pellets were sent to the NWRC Analytical Chemistry Unit in Fort Collins, CO, for confirmation of brodifacoum concentrations.
Statistical analyses
All statistical analysis was performed using the open access R environment for statistical computing (R Core Team 2018) and figures were produced using the package ggplot2 (Wickham 2009). Multiple logistic regression analysis was performed using the package lme4 (Bates et al. 2015). All statistical tests were two-tailed with significance levels of p < 0.05. Means are reported with ± 1 standard error of the estimate.
Results
Part 1: efficacy
The mean body mass of all mice at the initiation of the diet trials was 16.1 ± 2.9 g (range: 10.5–23.2 g). There was no significant difference in body mass of mice among cages (one-way ANOVA: F24,75 = 1.31, p = 0.2) or treatment groups (one-way ANOVA: F4,95 = 0.28, p = 0.9). No mortality occurred within the control or placebo treatment groups (see Appendix Table 5). The first mortalities for the low- and high-palatability treatments occurred four days after toxic bait was provided to the treatment groups, while the first mortality in the no-choice B25D treatment occurred after three days (Fig. 2). The average time to death of the eight mice (40%) that died in the low-palatability and high-palatability treatments was 6.9 ± 2.9 and 6.5 ± 1.9 days, respectively. The last mortality in the low-palatability treatment group occurred eleven days after exposure to toxic bait, and nine days in the high-palatability treatment. Ten days after exposure to B25D bait, all twenty (100%) of the mice in the no-choice treatment were dead, and the average time to death was 6.5 ± 1.9 days. There was no significant difference in the average time to death among the three different treatments (one-way ANOVA: F2,33 = 0.11, p = 0.89), but overall mortality was higher in the no-choice treatment group (100%) than both the low- and high-palatability treatments, which were both 40%.
Attrition of mice following exposure to experimental diets in Part 1 of the study. Mice were group housed 4 individuals to a cage and observed 14 days following initial exposure to Brodifacoum-25D Conservation (B-25D) bait and divided into a control group (open squares) and four treatment groups: two treatments consisting of a choice between toxic B-25D without pyranine and alternate diets of: high-palatability “mixed diet” (closed circles); low palatability “EPA challenge diet” (closed triangles); no choice trial with diet consisting of only toxic B-25D bait without pyranine (closed diamonds); and a placebo version of B-25 with pyranine treatment
Part 1: palatability
Because individual mice were not marked in the group housing cages (four mice per cage) and we were unable to monitor actual consumption of food, consumption estimates, and palatability scores are based on average consumption of bait and alternative food items for each cage in each treatment group (Table 2). When presented with an alternative food option, mice always consumed more of the alternative food than pellets of the non-toxic without pyranine or active without pyranine B-25D (Table 3), but there was no significant difference in the amount of alternative food consumed among treatment groups (one-way ANOVA: F2,12 = 1.37, p = 0.29). Regardless of the treatment group, palatability scores indicated that mice found the pellet bait less palatable than the alternate food items (i.e., all scores < 0.5). However, coefficients of variation for palatability scores revealed high variability among cages and treatment groups (range: 68–86%; Table 3).
Part 2: Bitrex efficacy and palatability trial
The mean body mass of all mice at the initiation of the trials was 15.9 ± 0.4 g (range: 11.5–24.0 g; see Appendix Table 6). There was no significant difference in body mass of mice among treatment groups (one-way ANOVA: F2,47 = 0.48, p = 0.62). Eight days following the exposure phase, one of the control animals died with no obvious cause of death (Table 4). This individual lost 1.5 g of weight (10.7% body weight), but otherwise looked and behaved normally at all the daily health monitoring checks. Otherwise, the first mortalities following the initial exposure to brodifacoum with and without Bitrex occurred at five days (range: 5–16) and eight days (range: 8–15), respectively (Fig. 3). Three individuals (two in the without Bitrex and one in the with Bitrex treatment groups) did not consume any bait and survived the trial. Counter to the guiding hypothesis for this test, there was higher mortality in the test group receiving bait with Bitrex (70%) than without (55%), though this difference was not statistically significant (z-test: X2 = 0.96, d.f. = 1, p = 0.33; Table 4). On average, individuals in the B-25D with Bitrex trial died sooner (8.4 ± 0.8 days) than individuals in the B-25D without Bitrex treatment (11.1 ± 0.6 days; two-sample t-test, t = − 2.19, d.f. = 23, p = 0.04; Table 4). Although the post-exposure monitoring period was extended to twenty days during this trial, as compared to the ten-day post-exposure monitoring period in Part 1, only three individuals died more than ten days post-exposure (two individuals fifteen and one individual sixteen days post-exposure).
Attrition of mice following exposure to experimental diets in Part 2 of the study. Mice were individually housed and observed 20 days following exposure to B-25D and were divided into two treatment groups given a choice between toxic B-25D with the bittering agent Bitrex (filled circles) and without (filled triangles) plus a control group (filled squares) that received the EPA challenge diet during the exposure period and LabDiet 5001 the rest of the study
When presented with an alternative food option, mice always consumed more of the alternative food than pellets of B-25D with or without Bitrex (Table 4), but there was no significant difference in the amount of alternative food consumed among treatment groups (two-sample t-test: t = − 0.05, d.f. = 38, p = 0.96) or the amount of bait consumed (two-sample t-test: t = 0.94, d.f. = 38, p = 0.35). Regardless of the treatment, palatability scores for all two-choice trials favored the alternate food items (i.e., all scores < 0.5).
Multiple logistic regression indicated no significant effect of initial body mass, sex, or treatment on the probability of survival versus death (all P > 0.52). On average, mice that died consumed more of the bait (3.51 ± 2.08 g) than individuals that survived (0.80 ± 1.96 g; t = 4.08, d.f. = 38, P < 0.001), including individuals that did consume some toxic bait, and received higher dosages of brodifacoum (mg of brodifacoum per kg of body mass; 5.45 ± 3.31 vs. 1.24 ± 2.87; two-sample t-test: t = 4.09, d.f. = 38, p < 0.001).
Chemical analyses
Analytical chemistry validated the concentrations of brodifacoum in the B-25D test materials at 0.00246% for Part 1 and 0.00287% for Part 2 (NWRC Analytical Services Reports 19-002 and 19-006), indicating that the product contained the nominal concentration of 0.0025% within a reasonable range of variability.
Discussion
Bait palatability
Regardless of testing protocols (group vs. individually housed), treatment groups (low vs. highly palatable alternate foods), or formulations (with or without pyranine or Bitrex), wild caught mice from Sand Island, MANWR found B-25D less palatable than both alternate diets (i.e., EPA challenge/low palatability diet or natural food items/high palatability). This was somewhat unexpected, given most two-choice trials have documented that wild caught mice generally find formulations of brodifacoum based rodenticides to be more palatable than commercially available rodent pellets (Cuthbert et al. 2011; O'Connor and Booth 2001; Pitt et al. 2011; Wheeler et al. 2019). Although these studies did not use the EPA challenge diet as the alternate food, another study that did also documented that wild-caught mice found the EPA challenge diet significantly more palatable than another formulation of a brodifacoum-based rodenticide (Cleghorn and Griffiths 2002). Unfortunately, it is unknown how the different formulations of commercially available “rodent pellets” purchased from pet or laboratory suppliers compare to the EPA challenge diet utilized in our study. Mice are known to prefer high-fat to high-carbohydrate diets (Romsos et al. 1982), so it may be that the EPA challenge diet, consisting of a loose mixture of 65% cornmeal, 25% rolled oats, 5% sugar, and 5% corn oil by weight, is simply more attractive to wild-caught mice than the pellet based rodenticide baits, most of which are comprised of grain-based bait materials (Fall 1982) and therefore are likely higher in carbohydrates.
If the diet and preferences of mice inhabiting Midway are shaped by the suite of potential food resources and their relative abundance unique to Midway, our assumptions about relative palatability of challenge diets could be incorrect. Generally house mice are primarily granivorous (Rowe 1973), but populations where plant based food sources are spatially and temporally limited show generalist and opportunistic feeding behaviors (Le Roux et al. 2002; Smith et al. 2002) and animal prey, particularly invertebrates, can form an important part of their diet (Copson 1986; Gleeson and Van Rensburg 1982; Le Roux et al. 2002; Rowe-Rowe et al. 1989; Smith et al. 2002). A recent study found that seabird-derived foods (e.g., deserted eggs and carcasses, discarded fish dropped by seabirds, and/or regurgitated pellets, and in some situations live chicks and adults) were a significant part of the diet of introduced field mice (Apodemus sylvaticus hirtensis) in and around seabird colonies on St. Kilda, Scotland, particularly during the breeding season (Anthony et al. 2020). The extremely high numbers and nearly year-round presence of breeding seabird populations on MANWR may provide an additional food source unique to Midway mice. If practical, we recommend that future eradication feasibility studies determine localized mouse diets before conducting comparative palatability studies to assist with selecting alternate diets. Future studies on mice in seabird colonies like Midway should include seabird-derived food items as a component, if not an entirely separate alternative diet.
Regardless, our results indicate that our a priori designation of low-palatability versus high-palatability was based on an apparently flawed assumption that the EPA challenge diet would be only minimally appealing to wild-caught mice from Sand Island. Instead, we found that it is at least as appealing as the most palatable mixed diet we could intuit. The EPA challenge diet was selected so that results could be more directly compared to the broader literature of previous rodenticide studies conducted for pesticide registrations. In hindsight, it would have been advisable for us to also evaluate a test group with only the standard LabDiet rodent maintenance pellets that are often used in field studies of relative palatability, for direct comparison to those studies.
While we could not measure individual consumption or palatability scores in the group housing study, cage averages of both consumption and palatability scores were not significantly different among cages but were highly variable. Rowe and Bradfield (1976) found similar variability in group housed families of mice, which could be due to social interactions. For example, mice can gain information about food via social transmission of food preferences (Galef 2002; Valsecchi and Galef 1989) and/or agonistic interactions between individuals could deter some individuals from accessing food (Forestier et al. 2018). Future group housing studies could measure agonistic interactions to determine if all individuals have equal access to the different diet alternatives.
Pyranine and Bitrex
Despite concerns that B-25D containing certain additives such as pyranine or Bitrex might reduce palatability to mice, we found no statistical difference in the amount of B-25D bait consumed containing either additive. While a previous laboratory efficacy study found that mice did not eat sufficient bait containing Bitrex to produce 100% mortality, they also noted that brodifacoum bait formulated with Bitrex was still effective in field trials (Kaukeinen and Buckle 1992).
Bait efficacy: no choice trial
Resistance to anticoagulant rodenticides is a worldwide phenomenon (Pelz et al. 2005) and has been documented in other insular mouse populations (Cuthbert and Hilton 2004). Usually the result of prolonged exposure to anticoagulant rodenticides (Bailey and Eason 2000), this is a possibility for the Midway mouse population, which has been exposed to anticoagulant rodenticides through intermittent control measures and a previous eradication of black rats (DIISE 2018). However, mortality in the no choice trial was 100% and the time to death following exposure was 6.5 days (± 1.9; range: 3–10). These results are similar to a no choice trial using a different formulation of brodifacoum documented by Cleghorn and Griffiths (2002) and indicate that the Midway mouse population is not resistant to brodifacoum.
Bait efficacy: two-choice trials
In both parts of the study, we found that the efficacy of B-25D for Sand Island mice in the presence of any alternative foods tested ranged from 40 to 70% mortality. Other studies assessing mouse susceptibility to different formulations of brodifacoum rodenticides using two-choice trials ranged from 50 to 100%, but most studies reported mortalities ≥ 90% (Cleghorn and Griffiths 2002; Cuthbert et al. 2011; O'Connor and Booth 2001; Pitt et al. 2011; Wheeler et al. 2019). As such, our mortality outcomes were lower than expected, but not dramatically different than ranges reported by other similar studies. Low efficacy is likely due to mice being more discriminant in their feeding preferences, actively foraging and consuming smaller quantities of food (Rowe 1973), and being more tolerant of anticoagulants including brodifacoum (Lund 1981; O'Connor and Booth 2001; Pitt et al. 2011; Wheeler et al. 2019). Perhaps another rodenticide product would have been more palatable, but B-25D was the only rodenticide evaluated in this study.
The lowest rates of efficacy came in Part 1 of the study, when only 40% of the mice in both the high- and low-palatability trials succumbed by the end of the ten-day monitoring period. This could be due to the nature of the group housing design, where social conditions could influence the ability of mice to ingest a lethal dose of bait (see palatability discussion above). Unfortunately, the large number of treatments and short window for testing necessitated group housing for this part of the study; to avoid uncertainty associated with possible artefacts of group housing, it is recommended that future studies reduce the number of test groups or increase the availability of resources to keep mice housed individually. However, with mice being social creatures, and conducting testing on recently captured wild mice, individual housing does not eliminate all behavioral sources of uncertainty.
The mean time to death did not differ among the three treatments, nor did the range of days to mortality (4–11). Unfortunately, logistical constraints limited post B-25D exposure monitoring for Part 1 of the study to only ten days, or 14 days from initial exposure. Previous work has reported that it can take up to 18 days for mice to die from brodifacoum poisoning (O'Connor and Booth 2001), but in our study all surviving individuals were euthanized at the end of the ten-day observation period so it is unknown whether more mice would have succumbed if the post-exposure monitoring period had been longer. We addressed this in Part 2 of the study, by extending the post-exposure monitoring to twenty days, and recommend that future studies extend the post-exposure observation period to at least twenty days as well.
In the second part of the study, there was no statistical difference in the efficacy of B-25D formulated with or without Bitrex (70% and 55% mortality, respectively), but the time to death was longer for individuals in the without-Bitrex treatment (8.4 days versus 11.1). However, the range of time to death for both treatments is within the range of means reported for mice in other experimental studies (Cleghorn and Griffiths 2002; Cuthbert et al. 2011; O'Connor and Booth 2001; Pitt et al. 2011; Wheeler et al. 2019). It is unclear why there was a difference in time to death between the two treatments; in addition to mere chance, one possible explanation could be rates of consumption. We did not detect a difference in the overall amount of bait consumed between the two formulations, but if consumption rates were lower or mice consumed smaller amounts of the without-Bitrex treatment per meal, this would mean that it would take them longer to ingest a lethal dose. Mice have been observed delaying consumption of toxic bait for several days in other trials (Cleghorn and Griffiths 2002; Pitt et al. 2011), but once this was accounted for, the time to death was similar to other studies (Cleghorn and Griffiths 2002). In this part of the study, we extended our post-monitoring observation period to twenty days to ensure that we could observe mortality in all moribund mice. Only three individuals died after the initial ten-day post-exposure monitoring period, with all individuals succumbing prior to eighteen days post exposure, similar to O'Connor and Booth (2001). If some individuals did not consume bait for several days (e.g., not until days three or four of the bait exposure period), it’s possible that some of the Part 1 survivors may have succumbed had we been able to monitor for a longer period of time. Because many of the mortalities failed to display any symptoms of toxicosis, it is difficult to know if some individuals delayed ingesting the bait but may have been nearing death. Because we did not record daily consumption rates, it is difficult to know if some individuals delayed eating bait. The value of daily bait consumption data must be carefully balanced against the labor demands, challenges of accurately calculating very small consumption amounts, and disturbance of mice during the exposure phase. In the end, insights gained will only be valuable if they can inform changes to operational protocols, such as prolonging bait exposure times to account for mice that are slow to start to feed on baits.
It is important to note that the poor performance of a specific toxic bait formulation in a laboratory setting does not necessarily indicate that the formulation will not be effective in a field setting. For example, laboratory efficacy trials of the same formulation of a diphacinone product yielded conflicting efficacy results (Pitt et al. 2011; Swift 1998), but appeared to perform satisfactorily in Hawaii (Spurr et al. 2003) and the Virgin Islands (Witmer et al. 2007). Given that mice in an eradication are unlikely to have ad libitum access to high quality alternative food sources, lower lab efficacy is not a predictor of eradication failure. The abundance and palatability of alternative foods during an eradication will be somewhere between none (100% mortality in no-choice trial) and high-palatability ad libitum (40–70% mortality in two-choice trials). Ultimately, the efficacy of any rodenticide relies on a combination of many different factors, most important of which are the toxicity of the rodenticide, the method of bait presentation or application (e.g., aerial, bait stations, hand broadcast), and the relative palatability and availability of the bait to the target species under the conditions when/where the bait is used. This emphasizes the importance of ensuring adherence to the fundamental eradication principles outlined by Cromarty et al. (2002).
Conclusions
Assessing the palatability and efficacy of any proposed formulation of a rodenticide on the target population prior to an eradication is an important first step in predicting the effectiveness of that formulation for the particular population. Laboratory studies can provide important insights into each of these factors but cannot infer the failure or success of the eradication. It is extremely difficult to recreate field conditions in the lab that will mimic how these factors combine in a field setting, making it important to remember that products performing below laboratory standards can perform adequately under field conditions, particularly if the project is implemented to a very high standard ensuring that fundamental pre-conditions for eradication success are achieved. Our results reinforce that the highest probability of a successful eradication requires a highly diligent and effective application of bait in compliance with principles of rodent eradications that errs on the side of ensuring that more bait than assumed is necessary is delivered into every potential mouse home range on the island. All variables (e.g., alternative foods such as garbage and foodstuffs, applying and monitoring of the bait) need to be managed with the highest degree of attention to detail by an experienced and committed field team dedicated to the eradication of mice from Sand Island.
Although toxic bait pellets may have proved to be of lower relative palatability than the EPA challenge diet and resulted in lower efficacy than expected, we do not believe that feasibility of eradication is compromised because the EPA challenge diet is used for laboratory comparisons and is not available to mice on Midway Atoll, thus highlighting the limitations of laboratory studies alone to infer probability of success. The probability of successfully removing mice from Midway Atoll depends on the relative availability and palatability of the bait compared to alternative food sources available to free-ranging mice, and mice that do not consume bait might be exposed to the rodenticide via other compartments of the food web such as invertebrates that consumed bait. Unfortunately, the a priori forecasting of the competitive palatability and availability of both alternative foods and brodifacoum in the food web over time is unreliable. Although we observed 100% mortality when there was no alternative diet, it is unlikely that free-ranging mice will have no alternative to bait; however, neither is it likely that all free-ranging mice will have ad libitum access to highly nutritious and potentially more preferred alternatives. We interpret this to add an imperative to determining mouse diets on Midway to inform risks to efficacy and implement risk minimization strategies such as removing alternative food resources in the landscape, in keeping with generally accepted practices and principles of rodent eradication.
Availability of data and material
Data provided in supplementary materials.
References
Angel A, Wanless RM, Cooper J (2009) Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biol Invasions 11:1743–1754. https://doi.org/10.1007/s10530-008-9401-4
Anthony WJB, Benjamin WW, Tom B et al (2020) Stable isotopes reveal the importance of seabirds and marine foods in the diet of St Kilda field mice. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-62672-x
Bailey CI, Eason CT (2000) Anticoagulant resistance in rodents. Department of Conservation, Wellington, New Zealand
Bates DM, Maechler M, Bolker BM et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Brooke MD, Bonnaud E, Dilley BJ et al (2018) Seabird population changes following mammal eradications on islands. Anim Conserv 21:3–12. https://doi.org/10.1111/acv.12344
Broome K, Golding C, Brown KP, et al. (2017) Rat eradication using aerial baiting: current agreed best practice used in New Zealand (Version 3.1). New Zealand Department of Conservation internal document DOC-29396, Wellington, New Zealand
Buckle A, Prescott C (2012) The current status of anticoagulant resistance in rats and mice in the UK. Report from the Rodenticide Resistance Action Group of the United Kingdom to the Health and Safety Executive., Vertebrate Pests Unit, The University of Reading, UK
Cleghorn M, Griffiths R (2002) Palatability and efficacy of Pestoff 20R bait on mice from Mokoia Island, Rotorua. Department of Conservation: Wellington, New Zealand
Copson GR (1986) The diet of the introduced rodents Mus musculus and Rattus rattus on subantarctic Macquarie Island. Aust Wildl Res 13:441–446. https://doi.org/10.1071/WR9860441
Croll DA, Maron JL, Estes JA et al (2005) Introduced predators transform subarctic islands from grasslands to tundra. Science 307:1959–1961. https://doi.org/10.1126/science.1108485
Cromarty P, Broome K, Cox A et al (2002) Eradication planning for invasive alien animal species on islands-the approach developed by the New Zealand Department of Conservation. In: Veitch CR, Clout MN (eds) Turning the tide: the eradication of invasive species: proceedings of the international conference on eradication of Island Invasives. IUCN, Gland, pp 85–91
Cuthbert R, Hilton G (2004) Introduced house mice Mus musculus: a significant predator of threatened and endemic birds on Gough Island, South Atlantic Ocean? Biol Conserv 117:483–489. https://doi.org/10.1016/j.biocon.2003.08.007
Cuthbert RJ, Visser P, Louw H et al (2011) Palatability and efficacy of rodent baits for eradicating house mice (Mus musculus) from Gough Island, Tristan da Cunha. Wildl Res 38:196–203. https://doi.org/10.1071/WR11016
DIISE (2018) The database of Island Invasive Species Eradications, developed by Island Conservation, Coastal Conservation Action Laboratory UCSC, IUCN SSC Invasive Species Specialist Group, University of Auckland and Landcare Research New Zealand. http://diise.islandconservation.org. Accessed 19 July 2020
Duhr M, Flint E, Hunter S et al (2019) Control of house mice preying on adult albatrosses at Midway Atoll National Wildlife Refuge. In: Veitch CR, Clout MN, Martin AR, Russel JC, West CJ (eds) Island invasives: scaling up to meet the challenge. Occasional Paper SSC no. 62. IUCN, Gland, pp 21–25
Fall MW (1982) Agricultural development and the ecology of rodent control. UNITAR conference on alternate strategies for desert development and management, vol 2. Pergamon Press, New York, pp 443–451
Forestier T, Féron C, Gouat P (2018) Transmission of food preference between unfamiliar house mice (Mus musculus domesticus) is dependent on social context. J Comp Psychol 132:268–279. https://doi.org/10.1037/com0000101
Galef BGJ (2002) Social learning of food preferences in rodents: rapid appetitive learning. Curr Protoc Neurosci. https://doi.org/10.1002/0471142301.ns0805ds21
Gleeson J, Van Rensburg P (1982) Feeding ecology of the house mouse Mus musculus on Marion Island. S Afr J Antarct Res 12:34–39
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: Their population biology and impacts on native species. Glob Ecol Conserv 3:607–627. https://doi.org/10.1016/j.gecco.2015.02.010
Harris DB (2009) Review of negative effects of introduced rodents on small mammals on islands. Biol Invasions 11:1611–1630. https://doi.org/10.1007/s10530-008-9393-0
Holmes ND, Griffiths R, Pott M et al (2015) Factors associated with rodent eradication failure. Biol Conserv 185:8–16. https://doi.org/10.1016/j.biocon.2014.12.018
Howald G, Donlan CJ, Galvan JP et al (2007) Invasive rodent eradication on islands. Conserv Biol 21:1258–1268. https://doi.org/10.1111/j.1523-1739.2007.00755.x
Island Conservation (2017) Midway Atoll field trial report, June/July 2017. Island Conservation, Santa Cruz, California, p 31
Island Conservation (2018) Midway Atoll field trial report, July and September 2018. Island Conservation, Santa Cruz, California, p 21
Jones HP, Holmes ND, Butchart SHM et al (2016) Invasive mammal eradication on islands results in substantial conservation gains. Proc Natl Acad Sci USA 113:4033–4038. https://doi.org/10.1073/pnas.1521179113
Kaukeinen DE, Buckle AP (1992) Evaluation of aversive agents to increase the selectivity of rodenticides with emphasis on denatonium benzoate (Bitrex) bittering agent. In: Proceedings of the vertebrate pest conference
Le Roux V, Chapuis JL, Frenot Y et al (2002) Diet of the house mouse (Mus musculus) on Guillou Island, Kerguelen archipelago, Subantarctic. Polar Biol. https://doi.org/10.1007/s003000100310
Lund M (1981) Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 87:101–107. https://doi.org/10.1017/s002217240006928x
MacKay JW, Russell JC, Murphy EC (2007) Eradicating house mice from islands: successes, failures and the way forward. In: Witmer GW, Pitt WC, Fagerstone KA (eds) Managing vertebrate invasive species. USPA/APHIS/WS, Fort Collins, USA
O'Connor CE, Booth LH (2001) Palatability of rodent baits to wild house mice. Sci Conserv 5–11
Pelz H-J, Rost S, Hünerberg M et al (2005) The genetic basis of resistance to anticoagulants in rodents. Genetics 170:1839–1847. https://doi.org/10.1534/genetics.104.040360
Pitt WC, Driscoll LC, Sugihara RT (2011) Efficacy of rodenticide baits for the control of three invasive rodent species in Hawaii. Arch Environ Contam Toxicol 60:533–542. https://doi.org/10.1007/s00244-010-9554-x
R Core Team (2018) R: A language and environment for statistical computing. R version 3.4.1 (2017-06-30) edn. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Reynolds MH, Berkowitz P, Courtot KN et al. (2012) Predicting sea-level rise vulnerability of terrestrial habitat and wildlife of the Northwestern Hawaiian Islands. US Geological Survey Report no. 2012–1182
Romsos DR, Bergen WG, Chee KM (1982) Protein intake regulation in adult obese (ob/ob) and lean mice: effects of nonprotein energy source and of supplemental tryptophan. J Nutr 112:505–513. https://doi.org/10.1093/jn/112.3.505
Rowe-Rowe DT, Green B, Crafford JE (1989) Estimated impact of feral house mice on sub-Antarctic invertebrates at Marion Island [Indian Ocean]. Polar Biol 9:457–460. https://doi.org/10.1007/BF00443233
Rowe FP (1973) Aspects of mouse behaviour related to control. Mammal Rev 3:58–63. https://doi.org/10.1111/J.1365-2907.1973.TB00172.X
Rowe FP, Bradfield A (1976) Trials of the anticoagulant rodenticide WBA 8119 against confined colonies of warfarin resistant house mice (Mus musculus L). J Hyg 77:427–431. https://doi.org/10.1017/S0022172400055819
Schneider BA (1982) Pesticide assessment guidelines: subdivision G, product performance. U.S. Environmental Protection Agency, Office of Pesticide and Toxic Substances, Springfield, VA, USA
Smith VR, Avenant NL, Chown SL (2002) The diet and impact of house mice on a sub-Antarctic island. Polar Biol 25:703–715. https://doi.org/10.1007/s00300-002-0405-8
Spurr E, Foote D, Perry CF et al (2003) Efficacy of aerial broadcast application of baits containing 0.005% diphacinone in reducing rat populations in Hawaiian forests. Pacific Island Ecosystems Research Center. US Geological Survey, Washington, DC
Swift C (1998) Laboratory bioassays with wild-caught black and Polynesian rats to determine minimum amounts of Ramik Green (0.005% diphacinone) and exposure times for field broadcast applications in Hawaii. MS thesis, University of Hawaii, Honolulu, HI
Towns DR, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions 8:863–891. https://doi.org/10.1007/s10530-005-0421-z
Valsecchi P, Galef JBG (1989) Social influences on the food preferences of House Mice (Mus musculus). Int J Comp Physiol 2:245–256
Varnham K (2010) Invasive rats on tropical islands: their history, ecology, impacts and eradication. RSPB
Veitch C, Clout M, Martin A et al (2019) Island invasives: scaling up to meet the challenge. Occasional Paper SSC. IUCN, Gland, p 734
Veitch C, Clout M, Towns D (2011) Island invasives: eradication and management. IUCN (International Union for Conservation of Nature), Gland
Wanless RM, Angel A, Cuthbert RJ et al (2007) Can predation by invasive mice drive seabird extinctions? Biol Lett 3:241–244. https://doi.org/10.1098/rsbl.2007.0120
Wheeler R, Priddel D, O’Dwyer T et al (2019) Evaluating the susceptibility of invasive black rats (Rattus rattus) and house mice (Mus musculus) to brodifacoum as a prelude to rodent eradication on Lord Howe Island. Biol Invasions 21:833–845. https://doi.org/10.1007/s10530-018-1863-4
Witmer GW, Boyd F, Hillis-Starr Z (2007) The successful eradication of introduced roof rats (Rattus rattus) from Buck Island using diphacinone, followed by an irruption of house mice (Mus musculus). Wildl Res 34:108–115. https://doi.org/10.1071/WR06006
Acknowledgements
This work was possible due to the logistical/permitting support from Papahānaumokuākea Marine National Monument staff. Midway Atoll NWR staff and volunteers, Kupu interns, and Chugach Federal Solutions’ staff provided logistical support in the field. We thank Bell Labs for producing a custom formulation of B-25D without Bitrex. Flights to/from Midway Atoll NWR were provided by Century Aviation. Refuge manager, Robert Peyton, helped to facilitate all aspects of the project conducted on the refuge. All animal use was performed according to protocol QA-2960, approved by the NWRC Institutional Animal Care and Use Committee.
Funding
This work was partially funded by Island Conservation through monies provided by the National Fish and Wildlife Foundation and other supporters, with additional support from the U.S. Fish and Wildlife Service, U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center (NWRC).
Author information
Authors and Affiliations
Contributions
All authors reviewed and approved the manuscript for submission. PJK and SRS analyzed the data and prepared the manuscript. ILL and RTS made substantial contributions to the initial manuscript draft. SRS, RTS, ILL, WJJ, ENF, KLG, and GRH contributed to study design development. JHP and KLG captured mice and performed animal care during acclimation period. ILL, PJK, and RTS ran bait trails and collected data. JHP and WJJ performed post-trial monitoring and animal care.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
National Wildlife Research Center IACUC approved study.
Consent for publication
All authors consent to have manuscript submitted for review and publication if accepted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Tables
5 and
6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kappes, P.J., Siers, S.R., Leinbach, I.L. et al. Relative palatability and efficacy of brodifacoum-25D conservation rodenticide pellets for mouse eradication on Midway Atoll. Biol Invasions 24, 1375–1392 (2022). https://doi.org/10.1007/s10530-021-02714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02714-1