Abstract
Objective
Evaluation of Nepeta cataria as a host with specific endogenous metabolite background for transient expression and metabolic engineering of secondary biosynthetic sequences.
Results
The reporter gene gfp::licBM3 as well as three biosynthetic genes leading to the formation of the cannabinoid precursor olivetolic acid were adopted to the modular cloning standard GoldenBraid, transiently expressed in two chemotypes of N. cataria and compared to Nicotiana benthamiana. To estimate the expression efficiency in both hosts, quantification of the reporter activity was carried out with a sensitive and specific lichenase assay. While N. benthamiana exhibited lichenase activity of 676 ± 94 μmol g−1 s−1, N. cataria cultivar ‘1000’, and the cultivar ‘Citriodora’ showed an activity of 37 ± 8 μmol g−1 s−1 and 18 ± 4 μmol g−1 s−1, respectively. Further, combinatorial expression of genes involved in cannabinoid biosynthetic pathway acyl-activating enzyme 1 (aae1), olivetol synthase (ols) and olivetolic acid cyclase (oac) in N. cataria cv. resulted presumably in the in vivo production of olivetolic acid glycosides.
Conclusion
Nepeta cataria is amenable to Agrobacterium-mediated transient expression and could serve as a novel chassis for the engineering of secondary metabolic pathways and transient evaluation of heterologous genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Engineering of metabolic pathways in alternative hosts holds a promising strategy to improve the production of valuable secondary metabolites or to generate new-to-nature compounds (Luo et al. 2015). Nicotiana benthamiana Domin has become both a model system and working horse for transient gene expression toward recombinant protein production as well as a platform for enzymatic pathway assembly and metabolite production (Bally et al. 2018). This fact also makes N. benthamiana an ideal exploration tool to develop novel metabolic engineering strategies, evaluate enzymatic cascades, and enable the fast and reliable testing of genetic constructs prior to a time-consuming stable transformation.
Although N. benthamiana has successfully been deployed to host complex metabolic routes and produce natural products of interest (Grzech et al. 2023) it has some limitations. Unintended glycosylation of pathway intermediates or end products (Gülck et al. 2020) or proteolysis of recombinant proteins (Grosse-Holz et al. 2017) has been described, which are an obstacle for high yields of any given product demanding complex and intensive engineering of the host genome (Dudley et al. 2022). Another factor that might limit the capacity of N. benthamiana is precursor supply, necessary for fueling the pipeline leading to secondary metabolites. The assembly of these specialized compounds rely on the supply of building blocks deviated from primary metabolism, and this is especially true for natural products containing terpenoid moieties within their structures, like monoterpenoid indole alkaloids (Geissler et al. 2016) or terpenophenolics (Schachtsiek et al. 2017). Hence, a strong endogenous supply of precursors like isopentenyl diphosphate (IPP) or geranyl diphosphate (GPP) in the heterologous host plant might be an advantage for the formation of novel metabolites. Moreover, sequestration and storage of newly produced metabolites seems to be a crucial aspect of high-level metabolite formation in host organisms. Especially glandular trichomes fulfill the role of specialized organs optimized to produce and store extreme amounts of natural products incompatible with the aqueous environment within a cell (Huchelmann et al. 2017).
Considering the reasons mentioned above, we identified Nepeta cataria L. (catnip) as a potential host plant for metabolic engineering efforts because of its chemotypes, rich in terpenoid constituents (nepetalactones and iridoids or citral derivatives (Said-Al Ahl et al. 2018)), and well-developed trichome system for the storage and secretion of the volatile components of essential oils. Hence, N. cataria could serve as a host for enzymatic routes relying on the terpenoid precursors. An important example of such a route is cannabinoid metabolic pathway, involving the integration of polyketides and terpene metabolites derived from the methylerythritol 4-phosphate pathway (Fellermeier et al. 2001; Gülk and Möller 2020; Supplementary Fig. 1).
Here we evaluate two chemotypes of N. cataria for their suitability for transient expression of heterologous genes, involved in a polyketide branch of cannabinoid biosynthesis, and accumulation of cannabinoid intermediates.
Materials and methods
Chemicals
Nerol, β-citronellol, geraniol and citral, used as authentic standards in GC–MS analysis, were procured from Sigma-Aldrich (St. Louis, MO, USA). Olivetolic acid (OA; Santa Cruz Biotechnology, Heidelberg, Germany) served as an authentic standard as well as a substrate in supplementation experiments. Hexanoic acid, used as precursor supply, was purchased from Carl Roth (Karlsruhe, Germany).
Bacterial strains and growth conditions
Escherichia coli TOP10 cells (Thermo Fisher Scientific, Waltham, USA), used for cloning, and Agrobacterium tumefaciens strain EHA105 and GV3101, applied in transient expression experiments, were maintained as described in Fräbel et al. (2016).
Cloning of DNA
To generate the genetic constructs used for transient transformation of N. cataria and N. benthamiana plants, restriction-ligation reactions were set up as reported by Sarrion-Perdigones et al. (2013). The domestication of aae1 (GenBank AFD33345.1), ols (GenBank AB164375.1) and oac (GenBank AFN42527.1), as well as the generation of A. tumefaciens EHA105 and GV3101 for transformation of plants is described in more detail in the supporting information. The constructs were assembled the way as described by Fräbel et al. (2016) utilizing the cauliflower mosaic virus 35S promoter (P35S) and the nopaline synthase terminator (TNos) for regulation of gene expression.
As a bifunctional reporter, we utilized an optimized version of licBM3 gene from Clostridium thermocellum (Ruminiclostridium thermocellum), coding for thermostable lichenase, in translational fusion with a synthetic GFP gene (NCBI acc. no KX458181; Gerasymenko et al. 2017).
Transient transformation of plants
All plants were grown in the greenhouse at 21 ± 2° C under 70% humidity and 16 h of illumination. Transient transformation of N. cataria and N. benthamiana plants was performed as previously described (Geissler et al. 2018). For reconstruction of the OA biosynthetic pathway, plants were additionally injected with 4 mM of hexanoic acid (solved in infiltration buffer (10 mM MES, 10 mM MgSO4) four days after initial infiltration with agrobacteria harboring aae1, ols and oac and harvested after 24 h of incubation.
Determination of lichenase activity
Total soluble proteins (TSP) were obtained as previously described (Geissler et al. 2018) using approximately 80 mg of plant material. For the determination of lichenase activity, assays were performed as described by (Gerasimenko et al., 2019).
GC–MS analysis of volatile compounds
For the analysis of volatiles accumulated in N. cataria plant material, the compounds were extracted with dichloromethane (DCM, Carl Roth, Karlsruhe, Germany) and the obtained samples measured by GC–MS using a DB-5MS column (30 m, 0.25 mm ID, 0.25 μm film thickness; Phenomenex, Aschaffenburg, Germany). Detailed parameters of sample preparation and GC–MS analysis are specified in the supplementary data.
Extraction of metabolites and HPLC–MS analysis of cannabinoid precursors
150 mg of frozen plant material of either N. benthamiana or N. cataria was homogenized by sonication in 200 μl of methanol/water (80:20, by vol.) for 30 min. The extracts were cleared by centrifugation at 17,000×g for 10 min and 4 °C and the supernatant injected to HPLC–MS analysis on an Agilent 1260 Infinity System using a Poroshell 120SB-C18 (3.0 × 150 mm, 2.7 μm; Agilent, Santa Clara, CA, USA) column, as previously described (Geissler et al. 2018). OA and OA glucosides were detected in negative selected ion monitoring (SIM) with selected m/z[M-H]- of 223.2 and 385.2, respectively.
Results and discussion
Agrobacterium-mediated transient transformation of N. cataria plants
In the first step, we wanted to evaluate the amenability of catnip to the infiltration with an Agrobacterium suspension. Secondly, heterologous gene expression needs to be verified by fluorescence microscopy and by enzymatic assay of lichenase activity. The upper six leaves of four-week-old plants were grouped in pairs according to the nodes from which they emerged. Plants were infiltrated either with A. tumefaciens (strain GV3101) or with the same strain harbouring the GFP::LicBM3 expression construct, providing a fusion of the gfp gene for fluorescence readout and the licBM3 gene encoding the enzyme lichenase (Supplementary Fig. 2) whose activity can be quantified after successful expression (Supplementary Fig. 3a). Additionally, a transcriptional unit harbouring the p19 protein to avoid post-transcriptional gene silencing was included in the vector. After seven days of incubation, all plants displayed barely any necrosis compared to non-infiltrated leaves (Supplementary Fig. 3b and c). Under UV light, an easily visible fluorescence could be observed in plants transformed with GV3101 carrying the GFP::LicBM3 expression construct (Supplementary Fig. 4). Since the transient transformation of N. cataria plants visually proved possible, a quantitative analysis of transformation efficiency was performed next. Lichenase activity was measurable in all three leaf pairs of both chemovars with 37 ± 8 μmol g−1 s−1 for the second leaf pair of N. cataria ‘1000’, and 18 ± 4 μmol g−1 s−1 activity in the first, and thus youngest, leaf pair of N. cataria ‘Citriodora’ (Fig. 1). Activity differed between leaves, but the difference was, however, not significant. For comparison, we took the data of N. benthamiana, four-week-old plants transformed with the same construct (Gerasymenko et al. 2019), where lichenase activity is most pronounced in the second to third upper leaves (650 ± 65 and 676 ± 94 μmol g−1 s−1, respectively, Fig. 1).
Lichenase activity in N. benthamiana (Gerasimenko et al., 2019; purple) and N. cataria leaves (cultivar ‘1000’ in teal and cultivar ‘Citriodora’ in lime) of different ages (1st-, the youngest leaf). In case of catnip plants, the two upper, the two middle and the two lower leaves were summarized as the first, second and third leaf, respectively, since they are emerging from the same node. Jittered dots represent the measured values, while the violines depict the data distribution for each leaf type
Although the expression level of the reporter protein in N. benthamiana was considerably higher compared to N. cataria, the general infiltration approach proved viable for both tested N. cataria varieties providing a detectable yield of the reporter protein. It cannot be excluded that other developmental stages of the plants could be more optimal concerning the expression levels since plant senescence alters gene expression and the endogenous phytohormone balance, which may influence susceptibility to viral/bacterial infection (Buchanan-Wollaston et al. 2005). Given the fact that older leaves were less amenable to the infiltration procedure and that the initial results confirmed the potential of catnip for rapid testing of genetic sequences for metabolite production, we continued with the established protocol.
Characterization of chemotypes within the different N. cataria cultivars
The Nepeta genus has been the subject of extensive research and studies on their phytochemical variations led to the definition of two main catnip chemotypes. One, with nepetalactones as the dominant compounds, and another with citral derivatives as major constituents (Said-Al Ahl et al. 2018). To evaluate the effect of the endogenous metabolic background on any heterologous pathway being introduced it was necessary to characterize the two utilized cultivars regarding their chemical profiles. Therefore, volatile compounds were extracted from the two studied N. cataria cultivars and analyzed by GC–MS. The results revealed two distinct chemotypes. The cultivar ‘Citriodora’ consisted exclusively of chemotype 1, containing citral derivatives like citronellol, neral, geraniol and geranial, while N. cataria cultivar ‘1000’ consisted exclusively of chemotype 2 containing diastereomers of nepetalactone in different proportions (Fig. 2).
GC − MS analysis of volatile compounds produced in two different N. cataria wildtype cultivars, consisting mainly of either citral derivatives (chemotype 1) or nepetalactone (chemotypes 2). 1, nerol; 2, β-citronellol; 3, neral; 4, geraniol; 5, geranial; 6, nepetalactone. The identities of metabolites 1–5 were confirmed by comparison of their MS spectra with authentic standards. Metabolite 6 was verified by comparison of its MS spectrum with reference outputs deposited in the National Institute of Standards and Technology (NIST) database and represents nepetalactone (mixture of diastereomers)
Production of olivetolic acid (OA) metabolites in transiently transformed N. cataria plants
For the introduction of a new pathway, we chose the enzymatic route derived from Cannabis sativa leading to the formation of phytocannabinoids. Although this pathway starts from an unusual precursor, hexanoic acid, it is well suited to probe our assumption of N. cataria capable of serving as a host for metabolic engineering efforts. As terpenophenolics, cannabinoids combine two biosynthetic routes, merging polyketides with isoprenoids (Schachtsiek et al. 2017). Here, we started with the first three enzymatic steps leading to the formation of olivetolic acid (OA), a key polyketide intermediate in the cannabinoid biosynthetic pathway.
As a prerequisite of the metabolic engineering efforts, we determined whether OA has toxic effects in catnip, as it has been shown for cannabigerolic acid (CBGA) and Δ9-tetrahydrocannibinolic acid (THCA) fed to C. sativa cell suspension cultures (Sirikantaramas et al. 2005). Within the same experimental setting, the suitability of the extraction method for isolation of OA could be evaluated. Therefore, N. cataria plants were injected with OA and incubated for 24 h. After extraction and analysis by HPLC–MS, not only OA could be detected, but also two other metabolites which eluted approximately 2 min earlier, exhibiting an m/z [M-H]− 385.2 (Fig. 3), suggesting that the extracted substances are the glycosylated forms of OA. This was also described for N. bethamiana transiently expressing the pathway genes to produce OA and whereupon the OA formed was glucosylated either at the hydroxy group at C-2 or at C-4 in planta (Gülck et al. 2020; Fig. 3).
HPLC–MS analysis of N. cataria cultivar ‘Citriodora’ and ‘1000’ as well as N. benthamiana plant extracts obtained from leaves infiltrated with olivetolic acid (OA). As a negative control (C) wildtype plants were solely infiltrated with infiltration buffer. In addition to OA, two additional metabolites with an m/z of 385.2 were identified. In comparison with the results obtained by Gülck et al. (2020) with OA infiltrated into Nicotiana benthamiana plants, the metabolites eluting might represent C-4 OA glucoside and C-2 OA glucoside
Next, metabolic engineering of OA biosynthesis had to be achieved by introduction of the pathway genes into the plant host. Therefore, genetic constructs were assembled for the accumulation of the desired enzymes (AAE1, OAC and OLS) and p19 suppressor of gene silencing in the cytosol (Supplementary Fig. 2; construct TOA1 and TOA2). For transient transformation of N. cataria plants, Agrobacterium suspensions harboring the constructs TOA1 and TOA2 were co-infiltrated, supplemented with 4 mM hexanoic acid four days post infiltration and harvested after another 24 h of incubation. Transiently transformed plants solely infiltrated with the infiltration buffer were used as a negative control. The extracted metabolites were injected to HPLC–MS and analyzed in terms of the production of OA and its glycosylated forms. Expression of the transgenes resulted in the detection of OA glycoside when supplemented with hexanoic acid (Fig. 4), indicating efficient assembling of the biosynthetic sequence in N. cataria. Detailed structure elucidation of the formed glycosides by NMR spectroscopy might be possible after preparative isolation of the products and is beyond the scope of this paper.
HPLC–MS analysis of N. cataria cultivar ‘1000’ (a) and ‘Citriodora’ b plant extracts obtained from leaves expressing the pathway genes required for the production of olivetolic acid (OA) and additional supplementation of 4 mM hexanoic acid. N. cataria wildtype plants infiltrated with OA served as a positive control. Plants infiltrated with infiltration buffer as well as plants injected with Agrobacterium harboring p19 and supplemented with hexanoic acid were used as negative controls. c Schematic description of the biosynthetic pathway leading to glucosylated olivetolic acid. AAE1, acyl-activating enzyme 1; OLS, olivetol synthase; OAC, olivetolic acid cyclase; UGTs, endogenous UDP-glycosyltransferases. Heterologously produced enzymes are boxed
In conclusion, using the polyketide branch of cannabinoid biosynthesis as an example, we have shown for the first time that catnip can be used as a chassis for the assembling of heterologous metabolic pathway and production of PNPs, representing an alternative to the model plant N. benthamiana.
References
Bally J, Jung H, Kayser O, Mortimer C, Naim F, Philips JG, Hellens R, Bombarely A, Goodin MM, Waterhouse PM (2018) The rise and rise of nicotiana benthamiana: a plant for all reasons. Annu Rev Phytopathol 25(56):405–426
Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signaling pathways between developmental and dark/starvation induced senescence in Arabidopsis. Plant J 42:567–585
Dudley QM, Jo S, Guerrero DAS, Chhetry M, Smedley MA, Harwood WA, Sherden NH, O’Connor SE, Caputi L, Patron NJ (2022) Reconstitution of monoterpene indole alkaloid biosynthesis in genome engineered Nicotiana benthamiana. Comm Biol 5:949
Fellermeier M, Eisenreich W, Bacher A, Zenk MH (2001) Biosynthesis of cannabinoids incorporation experiments with 13C-labeled glucoses. Eur J Biochem 268(6):1596–1604
Fräbel S, Krischke M, Staniek A, Warzecha H (2016) Recombinant flavin-dependent halogenases are functional in tobacco chloroplasts without co-expression of flavin reductase genes. Biotechnol J 11(12):1586–1594
Geissler M, Burghard M, Volk J, Staniek A, Warzecha H (2016) A novel cinnamyl alcohol dehydrogenase (CAD)-like reductase contributes to the structural diversity of monoterpenoid indole alkaloids in Rauvolfia. Planta 243(3):813–824
Geissler M, Volk J, Stehle F, Kayser O, Warzecha H (2018) Subcellular localization defines modification and production of Δ9-tetrahydrocannabinolic acid synthase in transiently transformed Nicotiana benthamiana. Biotechnol Lett 40(6):981–987
Gerasymenko IM, Sheludko YV (2017) Synthetic cold-inducible promoter enhances recombinant protein accumulation during Agrobacterium-mediated transient expression in Nicotiana excelsior at chilling temperatures. Biotechnol Lett 39(7):1059–1067
Gerasymenko I, Sheludko Y, Frabel S, Staniek A, Warzecha H (2019) Combinatorial biosynthesis of small molecules in plants: engineering strategies and tools. Methods Enzymol 617:413–442
Gülck T, Møller BL (2020) Phytocannabinoids: origins and Biosynthesis. Trends Plant Sci 25(10):985–1004
Gülck T, Booth JK, Carvalho Â, Khakimov B, Crocoll B, Motawia MS, Møller BL, Bohlmann J, Gallage NJ (2020) Synthetic biology of cannabinoids and cannabinoid glucosides in Nicotiana benthamiana and Saccharomyces cerevisiae. J Nat Prod 83(10):2877–2893
Grosse-Holz F, Kelly S, Blaskowski S, Kaschani F, Kaiser M, van der Hoorn RAL (2017) The transcriptome, extracellular proteome and active secretome of agroinfiltrated Nicotiana benthamiana uncover a large, diverse protease repertoire. Plant Biotechnol J 16(5):1068–1084
Grzech D, Hong B, Caputi L, Sonawane PD, O’Connor SE (2023) Engineering the biosynthesis of late-stage vinblastine precursors precondylocarpine acetate, catharanthine, tabersonine in Nicotiana benthamiana. ACS Synth Biol 12:27–34
Huchelmann A, Boutry M, Hachez C (2017) Plant glandular trichomes: natural cell factories of high biotechnological interest. Plant Physiol 175(1):6–22
Lai H, He J, Engle M, Diamond MS, Chen Q (2012) Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol J 10(1):95–104
Luo Y, Li B-Z, Liu D, Zhang L, Chen Y, Jia B, Zeng B-X, Zhao H, Yuan Y-J (2015) Engineered biosynthesis of natural products in heterologous hosts. Chem Soc Rev 44(15):5265–5290
Said-Al Ahl H, Naguib NY, Hussein MS (2018) Evaluation growth and essential oil content of catmint and lemon catnip plants as new cultivated medicinal plants in Egypt. Ann Agric Sci 63(2):201–205
Sarrion-Perdigones A, Vazquez-Vilar M, Palaci J, Castelijns B, Forment J, Ziarsolo P, Blanca J, Granell A, Orzaez D (2013) GoldenBraid 2.0: a comprehensive DNA assembly framework for plant synthetic biology. Plant Physiol 162(3):1618–1631
Schachtsiek J, Warzecha H, Kayser O, Stehle F (2017) Current perspectives on biotechnological cannabinoid production in plants. Planta Med 84(4):214–220
Sirikantaramas S, Taura F, Tanaka Y, Ishikawa Y, Morimoto S, Shoyama Y (2005) Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of glandular trichomes. Plant Cell Physiol 46(9):1578–1582
Supporting information
Supplementary Fig. 1 Schematic description of the cannabinoid biosynthesis pathway in Cannabis sativa. MEP, 2-C-methyl-D-erythritol-4-phosphate pathway; AAE1, acyl activating enzyme 1; OLS, olivetol synthase; OAC, olivetolic acid cyclase; CBGAS, cannabigerolic acid synthase; THCAS, Δ9-tetrahydrocannabinolic acid synthase; CBDAS, cannabidiolic acid synthase; CBCAS, cannabichromenic acid synthase.
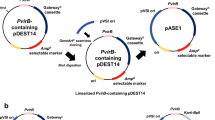
Supplementary Fig. 2—Schematic representation of the generated constructs within the GoldenBraid grammar (Sarrion-Perdigones et al. 2013). The capital letters show the four − nucleotide overhangs ensuring correct final orientation within the transcriptional unit (TU), while the numbers above the scheme represent standard GoldenBraid classes within the TU structure. P35S ATG, cauliflower mosaic virus (CaMV) 35S promoter with an integrated start codon ensuring cytosolic localization; TNos, nopaline synthase terminator; 8 × his:TNos, nopaline synthase terminator comprising an 8 × his-tag; aae1, acyl-activating enzyme 1; ols, olivetol synthase; oac, olivetolic acid cyclase; gfp::licBM3, lichenase in translational fusion to a synthetic GFP. Abbreviation of each construct is listed on the right. Boxes are not drawn to scale.
Supplementary Fig. 3 (a) Infiltration scheme of the upper six leaves (1a, 1b, 2a, 2b, 3a, 3b) of four-week-old N. cataria plants. (b) The infiltrated leaves showed very little necrosis at seven dpi compared to non-infiltrated leaves (c).
Supplementary Fig. 4 Fluorescence microscopy of N. cataria variety ‘Citriodora’ and ‘1000’ and N. benthamiana plants expressing gfp::licBM3 + P19. Infiltration of plants with untransformed Agrobacterium served as a negative control (C).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geissler, M., Neubauer, C., Sheludko, Y.V. et al. Nepeta cataria L. (catnip) can serve as a chassis for the engineering of secondary metabolic pathways. Biotechnol Lett (2024). https://doi.org/10.1007/s10529-024-03489-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10529-024-03489-w