Abstract
Objective
Salidroside is an important plant-derived aromatic compound with diverse biological properties. The main objective of this study was to synthesize salidroside from tyrosol using UDP-glucosyltransferase (UGT) with in situ regeneration of UDP-glucose (UDPG).
Results
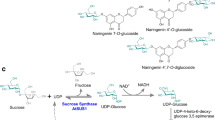
The UDP-glucosyltransferase 85A1 (UGT85A1) from Arabidopsis thaliana, which showed high activity and regioselectivity towards tyrosol, was selected for the production of salidroside. Then, an in vitro cascade reaction for in situ regeneration of UDPG was constructed by coupling UGT85A1 to sucrose synthase from Glycine max (GmSuSy). The optimal UGT85A1-GmSuSy activity ratio of 1:2 was determined to balance the efficiency of salidroside production and UDP-glucose regeneration. Different cascade reaction conditions for salidroside production were also determined. Under the optimized condition, salidroside was produced at a titer of 6.0 g/L with a corresponding molar conversion of 99.6% and a specific productivity of 199.1 mg/L/h in a continuous feeding reactor.
Conclusion
This is the highest salidroside titer ever reported so far using biocatalytic approach.





Similar content being viewed by others
References
Biswal S, Barhwal KK, Das D, Dhingra R, Dhingra N, Nag TC, Hota SK (2018) Salidroside mediated stabilization of Bcl-X-L prevents mitophagy in CA3 hippocampal neurons during hypoxia. Neurobiol Dis 116:39–52. https://doi.org/10.1016/j.nbd.2018.04.019
Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H (2000) Rhodiola rosea in stress induced fatigue—a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine 7:365–371. https://doi.org/10.1016/s0944-7113(00)80055-0
Fan B, Chen T, Zhang S, Wu B, He B (2017) Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside. Sci Rep 7:463. https://doi.org/10.1038/s41598-017-00568-z
Grech-Baran M, Syklowska-Baranek K, Pietrosiuk A (2015) Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola sp. in vitro cultures. Phytochem Rev 14:657–674. https://doi.org/10.1007/s11101-014-9368-y
Gutmann A, Bungaruang L, Weber H, Leypold M, Breinbauer R, Nidetzky B (2014) Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chem 16:4417–4425. https://doi.org/10.1039/c4gc00960f
He Q, Yin H, Jiang J, Bai Y, Chen N, Liu S, Zhuang Y, Liu T (2017) Fermentative production of phenolic glucosides by Escherichia coli with an engineered glucosyltransferase from Rhodiola sachalinensis. J Agric Food Chem 65:4691–4697. https://doi.org/10.1021/acs.jafc.7b00981
Li G, Lian J, Xue H, Jiang Y, Wu M, Lin J, Yang L (2020) Enzymatic preparation of pyruvate by a whole-cell biocatalyst coexpressing l-lactate oxidase and catalase. Process Biochem 96:113–121. https://doi.org/10.1016/j.procbio.2020.04.014
Li GS, Zhu FC, Wei PP, Gu FL, Xu QL, Ma MH (2022) Development of an Escherichia coli whole cell biocatalyst for the production of hyperoside. Biotechnol Lett 44:1073–1080. https://doi.org/10.1007/s10529-022-03285-4
Liu X, Li XB, Jiang JL, Liu ZN, Qiao B, Li FF, Cheng JS, Sun XC, Yuan YJ, Qiao JJ, Zhao GR (2018) Convergent engineering of syntrophic Escherichia coli coculture for efficient production of glycosides. Metab Eng 47:243–253. https://doi.org/10.1016/j.ymben.2018.03.016
Liu S, Xia Y, Yang H, Shen W, Chen X (2022) Rational chromosome engineering of Escherichia coli for overproduction of salidroside. Biochem Eng J. https://doi.org/10.1016/j.bej.2022.108474
Ma Y, Wang Y, Liu J, Lv F, Chen J, Zhou Z (2014) The effects of fruiting positions on cellulose synthesis and sucrose metabolism during cotton (Gossypium hirsutum L.) fiber development. PLoS ONE 9:e89476. https://doi.org/10.1371/journal.pone.0089476
Torrens-Spence MP, Pluskal T, Li F-S, Carballo V, Weng J-K (2018) Complete pathway elucidation and heterologous reconstitution of Rhodiola salidroside biosynthesis. Mol Plant 11:205–217. https://doi.org/10.1016/j.molp.2017.12.007
Xue F, Guo H, Hu Y, Liu R, Huang L, Lv H, Liu C, Yang M, Ma L (2016) Expression of codon-optimized plant glycosyltransferase UGT72B14 in Escherichia coli enhances salidroside production. Biomed Res Int. https://doi.org/10.1155/2016/9845927
Zhang X, Xie L, Long J, Xie Q, Zheng Y, Liu K, Li X (2021) Salidroside: a review of its recent advances in synthetic pathways and pharmacological properties. Chem-Biol Interact. https://doi.org/10.1016/j.cbi.2020.109268
Acknowledgements
This work was funded by the Open Fund of Anhui Engineering Laboratory for Conservation and Sustainable Utilization of Traditional Chinese Medicine Resources (TCMRPSU-2022-03), the Natural Science Foundation of Higher Education Institutions of Anhui Province (2023AH052649), the Fund of Generic Technology Research center for Anhui Traditional Chinese Medicine Industry (AHTCMGTRC-2023-09), the National Natural Science Foundation of China (32201979) and the University Research Project of Anhui Province (2022AH051682).
Funding
Open Fund of Anhui Engineering Laboratory for Conservation and Sustainable Utilization of Traditional Chinese Medicine Resources (TCMRPSU-2022-03), Natural Science Foundation of Higher Education Institutions of Anhui Province (2023AH052649), Fund of Generic Technology Research center for Anhui Traditional Chinese Medicine Industry (AHTCMGTRC-2023-09), National Natural Science Foundation of China (32201979), and University Research Project of Anhui Province (2022AH051682).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, G., Xu, Q., Hu, N. et al. Highly efficient biosynthesis of salidroside by a UDP-glucosyltransferase-catalyzed cascade reaction. Biotechnol Lett 46, 173–181 (2024). https://doi.org/10.1007/s10529-023-03453-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-023-03453-0