Abstract
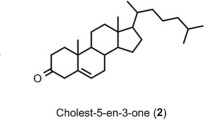
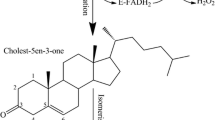
Cholesterol oxidase is industrially important as it is frequently used as a biosensor in food and agriculture industries and measurement of cholesterol. Although, most natural enzymes show low thermostability, which limits their application. Here, we obtained an improved variant of Chromobacterium sp. DS1 cholesterol oxidase (ChOS) with enhanced thermostability by random mutant library applying two forms of error-prone PCR (serial dilution and single step). Wild-type ChOS indicated an optimal temperature and pH of 70 ºC and pH 7.5, respectively. The best mutant ChOS-M acquired three amino acid substitutions (S112T, I240V and A500S) and enhanced thermostability (at 50 °C for 5 h) by 30%. The optimum temperature and pH in the mutant were not changed. In comparison to wild type, circular dichroism disclosed no significant secondary structural alterations in mutants. These findings show that error-prone PCR is an effective method for enhancing enzyme characteristics and offers a platform for the practical use of ChOS as a thermal-resistance enzyme in industrial fields and clinical diagnosis.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akbulut N, TuzlakoğluÖztürk M, Pijning T, İşseverÖztürk S, Gümüşel F (2013) Improved activity and thermostability of Bacillus pumilus lipase by directed evolution. J Biotechnol 164(1):123–129. https://doi.org/10.1016/j.jbiotec.2012.12.016
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20(4):470–475
Bloom JD, Arnold FH (2009) In the light of directed evolution: pathways of adaptive protein evolution. Proc Natl Acad Sci USA 106(1):9995. https://doi.org/10.1073/pnas.0901522106
Cirino PC, Mayer KM, Umeno D (2003) Generating mutant libraries using error-prone PCR directed evolution library creation. Springer, Cham, pp 3–9
Doukyu N (2009) Characteristics and biotechnological applications of microbial cholesterol oxidases. Appl Microbiol Biotechnol 83(5):825–837
Doukyu N, Shibata K, Ogino H, Sagermann M (2008) Purification and characterization of Chromobacterium sp. DS-1 cholesterol oxidase with thermal, organic solvent, and detergent tolerance. Appl Microbiol Biotechnol 80(1):59–70
Doukyu N, Shibata K, Ogino H, Sagermann M (2009) Cloning, sequence analysis, and expression of a gene encoding Chromobacterium sp. DS-1 cholesterol oxidase. Appl Microbiol Biotechnol 82(3):479–490. https://doi.org/10.1007/s00253-008-1775-9
Fazaeli A, Golestani A, Lakzaei M, Varaei SSR, Aminian M (2018) Expression optimization of recombinant cholesterol oxidase in Escherichia coli and its purification and characterization. AMB Express 8(1):1–9
Fazaeli A, Golestani A, Lakzaei M, RasiVaraei SS, Aminian M (2019) Expression optimization, purification, and functional characterization of cholesterol oxidase from Chromobacterium sp. DS1. PloS one 14(2):e0212217
Fazaeli A, Fana SE, Golestani A, Aminian M (2022) Improvement of thermostability of cholesterol oxidase from streptomyces Sp. SA-COO by random mutagenesis. Protein Expr Purif 191:106028. https://doi.org/10.1016/j.pep.2021.106028
Guan L, Gao Y, Li J, Wang K, Zhang Z, Yan S, Ji N, Zhou Y, Lu S (2020) Directed evolution of Pseudomonas fluorescens lipase variants with improved thermostability using error-prone PCR. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.01034
Imanaka T, Shibazaki M, Takagi M (1986) A new way of enhancing the thermostability of proteases. Nature 324(6098):695–697
Lee SY, Rhee HI, Tae WC, Shin JC, Park BK (1989) Purification and characterization of cholesterol oxidase from Pseudomonas sp. and taxonomic study of the strain. Appl Microbiol Biotechnol 31(5):542–546
MacLachlan J, Wotherspoon A, Ansell R, Brooks C (2000) Cholesterol oxidase: sources, physical properties and analytical applications. J Steroid Biochem Mol Biol 72(5):169–195
McCullum EO, Williams BA, Zhang J, Chaput JC (2010) Random mutagenesis by error-prone PCR In vitro mutagenesis protocols. Springer, Cham, pp 103–109
Moradpour Z, Ghasemian A (2016) Protein engineering of microbial cholesterol oxidases: a molecular approach toward development of new enzymes with new properties. Appl Microbiol Biotechnol 100(10):4323–4336
Nishiya Y, Harada N, Teshima SI, Yamashita M, Fujii I, Hirayama N, Murooka Y (1997) Improvement of thermal stability of Streptomyces cholesterol oxidase by random mutagenesis and a structural interpretation. Protein Eng 10(3):231–235
Pollegioni L, Piubelli L, Molla G (2009) Cholesterol oxidase: biotechnological applications. FEBS J 276(23):6857–6870
Rubin-Pitel SB, Cho CM, Chen W, Zhao H (2007) Directed evolution tools in bioproduct and bioprocess development bioprocessing for value-added products from renewable resources. Elsevier, Amsterdam, pp 49–72
Sagermann M, Ohtaki A, Newton K, Doukyu N (2010) Structural characterization of the organic solvent-stable cholesterol oxidase from Chromobacterium sp. DS-1. J Struct Biol 170(1):32–40
Stadtman TC, Cherkes A, Anfinsen CB (1954) Studies on the microbiological degradation of cholesterol. J Biol Chem 206(2):511–523
Vartanian JP, Henry M, Wain-Hobson S (1996) Hypermutagenic PCR involving all four transitions and a sizeable proportion of transversions. Nucleic Acids Res 24(14):2627–2631
Wakarchuk WW, Sung WL, Campbell RL, Cunningham A, Watson DC, Yaguchi M (1994) Thermostabilization of the Bacillus circulans xylanase by the introduction of disulfide bonds. Protein Eng Des Sel 7(11):1379–1386
Wang X, Minasov G, Shoichet BK (2002) Evolution of an antibiotic resistance enzyme constrained by stability and activity trade-offs. J Mol Biol 320(1):85–95. https://doi.org/10.1016/S0022-2836(02)00400-X
Whitmore L, Wallace BA (2008) Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89(5):392–400
Xu Z, Cen YK, Zou SP, Xue YP, Zheng YG (2020) Recent advances in the improvement of enzyme thermostability by structure modification. Crit Rev Biotechnol 40(1):83–98
Funding
This study was funded by Tehran University of Medical Sciences (Grant Number 30859).
Author information
Authors and Affiliations
Contributions
Study conception and design performed by MA. Investigation, material preparation, data collection and analysis were performed by AF, MA and SEF. The original draft of the manuscript was written by SEF and all authors commented on previous versions of the manuscript. All authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was reviewed and approved by the Ethics Committee of the Tehran University of Medical Sciences (IR.TUMS.REC.1395.2376).
Research involving human and animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebrahimi Fana, S., Fazaeli, A. & Aminian, M. Directed evolution of cholesterol oxidase with improved thermostability using error-prone PCR. Biotechnol Lett 45, 1159–1167 (2023). https://doi.org/10.1007/s10529-023-03401-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-023-03401-y