Abstract
Objectives
In our previous study, citrate was used as auxiliary energy substance for improving cAMP fermentation performance, however, the regulation mechanism of citrate on improved cAMP contents was not clear. To elucidate the regulation mechanism, cAMP fermentations with/without citrate addition were conducted in a 7 L fermentor using Arthrobacter sp. CCTCC 2013431 and assays on key enzymes activities, energy metabolism level, amino acids contents and peroxidation level were performed.
Results
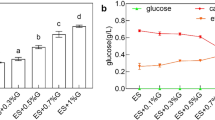
With 3 g/L-broth sodium citrate added, cAMP concentration and conversion yield from glucose reached 4.34 g/L and 0.076 g/g which were improved by 30.7% and 29.8%, respectively, when compared with those of control. Citrate changed carbon flux distribution among different routes and more carbon flux was directed into pentose phosphate pathway beneficial to cAMP synthesis. Meanwhile, energy metabolism together with precursor amino acids levels were improved significantly owing to strengthened metabolic intensity of tricarboxylate cycle by exogenous citrate utilization which provided energy and substance basis for cAMP production. Moreover, higher glutamate synthesis and oxidative stress caused by citrate addition consumed excessive NADPH derived from pentose phosphate pathway by which feedback suppression for pentose phosphate pathway was relieved efficiently.
Conclusion
Citrate promoted cAMP fermentation production by Arthrobacter sp. CCTCC 2013431 due to enhanced precursor amino acids, energy metabolism level and relieved feedback suppression for pentose phosphate pathway.





Similar content being viewed by others
References
CaoY ME, Shi ZP (2013) A novel metabolic model incorporating directed signal flow diagram with enzymatic activities data for evaluating glutamate yield in glutamate fermentation. Biochem Eng J 77:136–146
Chen H, Cao X, Zhu NQ et al (2020) A stepwise control strategy for glutathione synthesis in Saccharomyces cerevisiae based on oxidative stress and energy metabolism. World J Microb Biot 36(8):177–182
Chen XC, Song H, Fang T et al (2010a) Enhanced cyclic adenosine monophosphate production by Arthrobacter A302 through rational redistribution of metabolic flux. Bioresource Techn 101:3159–3163
Chen Y, Li SY, Xiong J et al (2010b) The mechanisms of citrate on regulating the distribution of carbon flux in the biosynthesis of uridine 5’-monophosphate by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 86:75–81
Chen Y, Liu Q, Chen XC et al (2015) Redirecting metabolic flux in Saccharomyces cerevisiae through regulation of cofactors in UMP production. J Ind Microbiol Biotechnol 42:577–583
Comasio A, Harth H, Weckx S et al (2019) The addition of citrate stimulates the production of acetoin and diacetyl by a citrate-positive Lactobacillus crustorum strain during wheat sourdough fermentation. Int J Food Microbiol 289:88–105
Gu Y, Tan H, Yuan LN et al (2021) Physiological mechanisms for enhanced cyclic adenosine monophosphate biosynthesis by sodium fluoride in Arthrobacter sp. Biotechnol Bull 37(5):108–116
Kang TS, Korber DR, Tanaka T (2013) Contributions of citrate in redox potential maintenance and ATP production: metabolic pathways and their regulation in Lactobacillus panis PM1. Appl Microbiol Biotechnol 97:8693–8703
Lelle M, Otte M, Thon S et al (2019) Chemical synthesis and biological activity of novel brominated 7-deazaadenosine-3’, 5’-cyclic monophosphate derivatives. Bioorgan Med Chem 27(8):1704–1713
Li S, Ji J, Hu S et al (2020) Enhancement of ε-poly-L-lysine production in Streptomyces griseofuscus by addition of exogenous astaxanthin. Bioproc Biosyst Eng 43(10):1813–1821
Li ZG, Chen BF, Fang ZB et al (2018) A novel fermentation process for cyclic adenosine monophosphate production based on citrate coupling hypoxanthine addition in pulses. Food Ferment Ind 44(11):154–158
Li ZG, Gu Y, Chen BF et al (2021) Physiological mechanism for enhanced cyclic adenosine monophosphate biosynthesis by sodium nitrate in Arthrobacter sp. CCTCC M2013431. Sci Technol Food Ind 42(1):115–120
Liu Q, Wu C, Huang S et al (2018) Decreased hyperpolarization-activated cyclic nucleotide-gated channels are involved in bladder dysfunction associated with spinal cord injury. Int J Mol Med 41(5):2609–2618
Niu HQ, Wang JZ, Zhuang W et al (2018) Comparative transcriptomic and proteomic analysis of Arthrobacter sp CGMCC 3584 responding to dissolved oxygen for cAMP production. Sci Rep. https://doi.org/10.1038/s41598-017-18889-4
Niu HQ, Sun XZ, Song J et al (2020) Knockout of pde gene in Arthrobacter sp. CGMCC 3584 and transcriptomic analysis of its effects on cAMP production. Bioproc Biosyst Eng 43(5):839–850
Sholokh A, Klussmann E (2021) Local cyclic adenosine monophosphate signalling cascades-roles and targets in chronic kidney disease. Acta Physiol. https://doi.org/10.1111/apha.13641
Wang A, Tian W, Cheng L et al (2020) Enhanced ε-Poly-L-Lysine production by the synergistic effect of ε-Poly-L-Lysine synthetase overexpression and citrate in Streptomyces albulus. Front Bioeng Biotech. https://doi.org/10.3389/fbioe.2020.00288
Wang YL, Wang DH, Wei GY et al (2013) Improved co-production of S-adenosylmethionine and glutathione using citrate as an auxiliary energy substrate. Bioresource Techn 131:28–32
Xia J, Xu ZX, Xu H et al (2014) The regulatory effect of citric acid on the co-production of poly (ε-lysine) and poly (L-diaminopropionic acid) in Streptomyces albulus PD-1. Bioprocess Biosyst Eng 37:2095–2103
Zeng X, Chen XS, Gao Y et al (2015) Continuously high reactive oxygen species generation decreased the specific ε-poly-L-lysine formation rate in fed-batch fermentation using glucose and glycerol as a mixed carbon source. Process Biochem 50(12):1993–2003
Zeng X, Chen XS, Ren XD et al (2016) Improved ε-poly-L-lysine productivity partly resulting from rapid cell growth in cultures using a glucose-glycerol mixed carbon source. Eng Life Sci 16(5):1–10
Zhou J, Liu L, Chen J (2010) Improved ATP supply enhances acid tolerance of Candida glabrata during pyruvic acid production. J Appl Microbiol 110:44–53
Zhu Q, Zhuang W, Niu H et al (2018) Affinity induced immobilization of adenylate cyclase from the crude cell lysate for ATP conversion. Colloid Surface B 164:155–164
Funding
This work was financially supported by the Science and Technology Breakthrough Plan of Henan Province (192102210193) and the Doctoral Scientific Research Foundation of Henan institute of science and technology (2015006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Chen, B., Gu, Y. et al. Enhanced endogenous amino acids and energy metabolism level for cAMP biosynthesis by Arthrobacter sp. CCTCC 2013431 with citrate as cosubstrate. Biotechnol Lett 43, 1989–1999 (2021). https://doi.org/10.1007/s10529-021-03170-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03170-6