Abstract
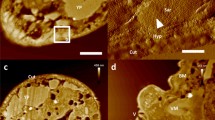
Atomic force microscopy (AFM) is a sophisticated imaging tool with nanoscale resolution that is widely used in structural biology, cell biology, and material science, among other fields. However, to date it has rarely been applied to the study of aquatic animals, especially on one of the main cultured species, shrimp. One reason for this is that no shrimp cell line established until now, primary cell is fragile and difficult to be studied under AFM. In this study, we used AFM to image three different types of biological material from shrimp (Litopenaeus vannamei) in air, including hemocytes and two associated pathogens. Without obvious deformations when the cells were imaged in air and in the case for the haemocytes and the cells were fixed as well. The result suggests hydrophobic glass coverslips are a suitable substrate for adhesion of these samples. The method described here can be applied to the preparation of other fragile biological samples from aquatic animals for high-resolution analyses of host–pathogen interactions and other basic physiological processes.



Similar content being viewed by others
References
Alonso JL, Goldmann WH (2003) Feeling the forces atomic microscopy in cell biology. Life Sci 72:2553–2560
Dubrovin EV, Kirikova MN, Novikov VK, Sgalari G, MacKintosh FC, Carrascosa JL, Schmidt CF, Wuite GJ (2004) Study of the peculiarities of adhesion of tobacco mosaic virus by atomic force microscopy. Colloid J 66(6):673–678
Dubrovin EV, Drygin YF, Novikov VK, Yaminsky IV (2007) Atomic force microscopy as a tool of inspection of viral infection. Nanomedicine 3(2):128–131
Fotiadis D, Scheuring S, Müller SA, Engel A, Müller DJ (2002) Imaging and manipulation of biological structures with the AFM. Micron 33:385–397
Francis LW, Lewis PD, Wright CJ, Conlan RS (2010) Atomic force microscopy comes of age. Biol Cell 102:133–143
Galloway JM, Senior L, Fletcher JM, Beesley JL, Hodgson LR, Harniman RL, Mantell JM, Coombs J, Rhys GG, Xue WF, Mosayebi M, Linden N, Liverpool TB, Curnow P, Verkade P, Woolfson DN (2017) Bioinspired silicification reveals structural detail in self-assembled peptide cages. ACS Nano 12(2):1420–1432
Lei Y, Chen HY, Dai HP, Zeng ZR, Lin Y, Zhou FM, Pang DW (2008) Electroless-plated gold films for sensitive surface plasmon resonance detection of white spot syndrome virus. Biosens Bioelectron 23(7):1200–1207
Li C, Gao XX, Huang J, Liang Y (2016) Studies of the viral binding proteins of shrimp BP53, a receptor of white spot syndrome virus. J Invertebr Pathol 134:48–53
Liang Y, Xu ML, Wang XW, Gao XX, Cheng JJ, Li C, Huang J (2015) ATP synthesis is active on the cell surface of the shrimp Litopenaeus vannamei and is suppressed by WSSV infection. Virol J 12:49
Liashkovich I, Hafezi W, Kühn JE, Oberleithner H, Kramer A, Shahin V (2008) Exceptional mechanical and structural stability of HSV-1 unveiled with fluid atomic force microscopy. J Cell Sci 121(14):2287–2292
Lightner DV, Hasson KW, White BL, Redman RM (1998) Experimental infection of western hemisphere penaeid shrimp with Asian white spot syndrome virus and Asian yellow head virus. J Aquat Anim Health 10:271–281
Liu QH, Zhang XL, Ma CY, Liang Y, Huang J (2009) VP37 of white spot syndrome virus interact with shrimp cells. Lett Appl Microbiol 48:44–50
Liu YJ, Wu JL, Chen H, Hew CL, Yan J (2010) DNA condensates organized by the capsid protein VP15 in White Spot Syndrome Virus. Virology 408(2):197–203
Liu J, Song XL, Liu L, Chai PC, Huang J (2012) Effects of digestive tract probiotics on immune enzyme activity and anti-WSSV ability of Litopenaeus vannamei. J Fish China 36(3):444–450
Liu QH, Ma FF, Guan GK, Wang XF, Li C, Huang J (2015) White spot syndrome virus VP51 interact with ribosomal protein L7 of Litopenaeus vannamei. Fish Shellfish Immunol 44:382–388
Ma FF, Liu QH, Guan GK, Li C, Huang J (2014) Arginine kinase of Litopenaeus vanname involved in white spot syndrome virus infection. Gene 539:99–106
Ma CY, Gao Q, Liang Y, Li C, Liu C, Huang J (2016) Shrimp arginine kinase being a binding protein of WSSV envelope protein VP31. Chin J Oceanol Limnol 34(6):1287–1296
Marchante R, Beal DM, Koloteva-Levine N, Purton TJ, Tuite MF, Xue WF (2017) The physical dimensions of amyloid aggregates control their infective potential as prion particles. eLife 6:27109
Mayer MJ, Juodeikis R, Brown IR, Frank S, Palmer DJ, Deery E, Beal DM, Xue WF, Warren MJ (2016) Effect of bio-engineering on size, shape, composition and rigidity of bacterial microcompartments. Sci Rep 6:36899
Ratanavalachai TC, William WA (1996) Effects of reactive oxygen species (ROS) modulators, TEMPOL and catalase, on methoxyacetaldehyde (MALD)-induced chromosome aberrations in Chinese hamster ovary (CHO)-AS52 cells. Mutat Res 357:25–33
Roos WH (2011) How to perform a nanoindentation experiment on a virus. Methods Mol Biol 783:251–264
Sritunyalucksana K, Wannapapho W, Lo CF, Flegel TW (2006) PmRab7 is a VP28- binding protein involved in white spot syndrome virus infection in shrimp. J Virol 80:10734–10742
Sun Y, Liu F, Song XL, Mai KS, Li YH, Huang J (2012) Effects of adding probiotics in the feed on non-specific immune gene expression and disease resistance of Litopenaeus Vannamei. Oceanol Limnol Sin 43(4):845–851
Tamayo J (2003) Structure of human chromosomes studied by atomic force microscopy. J Struct Biol 141:198–207
Tamiya E, Saito M, Iwabuchi S, Morita Y (2003) Nanoscopic imaging of human chromosomes via a scanning near-field optical/atomic-force microscopy (SNOAM). Sci Technol Adv Mater 4:61–67
Xie XX, Li HY, Xu LM, Yang F (2005) A simple and efficient method for purification of intact white spot syndrome virus (WSSV) viral particles. Virus Res 108:63–67
Yang F, Wang W, Chen RZ, Xu X (1997) A simple and efficient method for purification of prawn baculovirus DNA. J Virol Methods 67:1–4
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 31302233); Special Scientific Research Funds for Central Non-Profit Institutes, Yellow Sea Fisheries Research Institute (Grant No. 20603022018014); the projects under Central Public-interest Scientific Institution Basal Research Fund, CAFS (Grant No: 2017HY-ZD1002) and China Agriculture Research System (Grant No. CARS-47).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Liang, Y., Xu, M. et al. Imaging aquatic animal cells and associated pathogens by atomic force microscopy in air. Biotechnol Lett 41, 1105–1110 (2019). https://doi.org/10.1007/s10529-019-02720-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02720-3