Abstract
The questions about why and how senescence occurs in the wild are among the most pertinent ones in evolutionary ecology. Telomere length is a commonly used marker for aging, while other biomarkers of aging have received considerably less attention. Here we studied how another potent indicator of aging—skin pentosidine concentration—relates to age and blood telomere length in a long-lived seabird with well-documented reproductive senescence. We found no associations between telomere length, skin pentosidine and chronological age in male common gulls (Larus canus), aging from 2 to 30 years. However, the variance in telomere length was 4.6 times higher among the birds older than 13 years, which hints at relaxed selection on telomere length among the birds that have passed their prime age of reproduction. These results suggest that physiological and chronological ages may be largely uncoupled in our study system. Furthermore, our findings do not support a hypothesis about the presence of a common physiological factor (e.g., such as oxidative stress) that would cause covariation between two independent markers of aging.
Similar content being viewed by others
Introduction
Performance of long-lived vertebrates declines in old age. This phenomenon of senescence is widespread in natural populations of animals and the possible causes range from pleiotropic effects of genes to mutation accumulation (reviewed by Nussey et al. 2013). Most studies of aging in the wild have focused on life-history traits like survival, reproduction or body mass, the pattern of age-specific changes in biochemical and haematological parameters remains virtually unknown to date for any population of vertebrate living in the wild (Jego et al. 2014).
In recent years, senescence has been increasingly associated with the rate of attrition of telomeres due to oxidative stress (Monaghan and Haussmann 2006; Haussmann and Marchetto 2010). Telomeres are the protective DNA sequences at the ends of chromosomes that progressively shorten in proliferative somatic cells. Thus, telomere length could be an indicator of a cell’s replicative age. Telomeres are highly sensitive to oxidative damage (Houben et al. 2008). Therefore, telomere shortening could provide a marker system to assess the overall effect of oxidative damage as an individual ages. However, the proof that oxidative damage to telomeres is particularly high in senescent cells mostly originates from studies of cell cultures (von Zglinicki 2002). The evidence about associations between telomere length and oxidative stress-related diseases is controversial (von Zglinicki and Martin-Ruiz 2005) and originates from studies of knockout mice (but see Asghar et al. 2015 and Ilmonen et al. 2008) and humans in industrialised societies, which complicates the interpretation in an evolutionary context. Information about the hypothesized interconnections between telomere length, oxidative stress and senescence in wild animals is scarce (Monaghan 2014). One reason for this is a shortage of suitable methods for assessment of individually consistent levels of oxidative damage (Kilk et al. 2014; Meitern et al. 2013; Sepp et al. 2012).
We measured two markers of oxidative damage, pentosidine and telomere length, in a long-lived seabird. Pentosidine (Ps) is a biomarker of both the glycative and oxidative damage to proteins. It is formed continuously under normal circumstances but more rapidly under a variety of stresses, especially under oxidative stress and hyperglycemia. The glycation theory of aging suggests that the modification of proteins by glucose leads to the gradual crosslinking, polymerization and fluorescence changes in collagen that are characteristic of aging, leading ultimately to the structural and functional deterioration of tissues (Iqbal et al. 1999). Pentosidine accumulates throughout the lifetime of an individual, which makes it a useful biomarker of chronological age that has been validated in numerous avian species (Chaney et al. 2003; Fallon et al. 2006; Cooey et al. 2010). This study involves male common gulls (Larus canus), breeding in a colony located on an islet at West coast of Estonia. The age of breeders ranges from 2 to 33 years. Reproductive success (probability of producing recruits) increases until the 10th breeding year (i.e. until the chronological age of 13–14 years) and decreases thereafter (Rattiste 2004). Annual fitness, a metric that integrates survival and reproduction, exhibits a senescent decline (Brommer et al. 2009).
The study aims to test the following predictions: (1) Pentosidine accumulation in skin predicts chronological age as in other bird species. (2) The length of telomeres in blood cells correlates with age. Here we had no directional prediction because both positive and negative correlations are feasible under the hypotheses that telomere length is related to aging (Haussmann and Mauck 2008; Holmes and Martin 2009). (3) Pentosidine levels correlate negatively with telomere length. To verify that the subsample of individuals measured is represenative to general age-dependent pattern of reproduction, we also tested for the presence of a concave relationship between egg laying date and age. In common gulls clutch size is invariant (three eggs), and laying date is thus presumably a key reproductive trait in this species. We considered the date of laying of the first egg as a proxy of male condition because there is a strong selection for early laying in this population and female’s laying date depends on the male’s ability to arrive early to the colony and quickly establish and defend a breeding site, his ability for courtship feeding, and his timing of spermatogenesis and copulation prowess (Brommer and Rattiste 2008). Previous studies have indicated that in this colony, breeding experience (a proxy of age) of males affects laying date and laying earlier results with higher recruitment (Brommer and Rattiste 2008).
Methods
Sampling procedure
The data were collected in Kakrarahu, a 3.7 ha islet situated in Matsalu National Park, Estonia (58°46′N, 23°26′E) where the monitoring of common gulls started in 1962. On average 94 % of nest owners are identified each year (Rattiste 2004; Brommer et al. 2009). The subsample was chosen to be as representative as possible of the overall age variation in this colony. The age of males in this study varied from 2 to 30 years. On average, males start to breed at 3.2 years and breed for 5–6 years, but oldest bird in the study area has bred for 30 years (age 33 years). Annual survival of adult birds is 86–93 % and therefore, only 20 % of common gulls breed for more than 10 years (Rattiste 2004). In the longitudinal analysis, the breeding success of common gulls increased until the tenth breeding year and started to decline thereafter (Rattiste 2004), therefore, birds aged 15+ years can be considered senescent (however, given the substantial differences in lifespan, the rate of senescence seems to be highly variable in this colony). The birds aged 15+ years in our sample therefore do not represent the average bird from this colony, but rather birds that outlive their companions by a wide margin. Male common gulls were caught from the nests with spring traps on 20–21 May and on 6 June 2013. Samples were collected under manual restraint. Birds were sampled within 5 min of capture and released after haemostasis was achieved. Handling of individual birds usually took up to 10 min.
A skin biopsy was taken from the inner patagium, where there was minimal vasculature. Only one biopsy per bird was taken. Any feathers obscuring the site were removed and the skin was cleaned with 70 % ethanol. A subcutaneous injection of 0.05 ml lignocaine (diluted 1:1 with 0.9 % sodium chloride solution) was used to provide local anaesthesia to the biopsy site, and to raise the thin epidermal layer from the underlying tissue. The skin was stretched so that it was taut and a disposable 6 mm diameter biopsy punch (Paramount Surgimed Ltd, New Delhi, India) was used to penetrate the epidermal layer. Atraumatic forceps and scissors were used to remove the skin sample and place it into an Eppendorf tube with distilled water. The tube was placed in liquid nitrogen. After haemostasis was achieved, Vetbond® Tissue Adhesive was used to seal the defect.
Blood sample was taken from the brachial vein using blood lancets. Blood was collected into 200 μl Microvette tubes with EDTA as an anticoagulant. 50 μl of blood was then transferred to an Eppendorf tube and mixed with SET buffer (0.15 M NaCl, 20 mM TrisHCl, 1 mM EDTA, pH 8.0). The tube was placed in liquid nitrogen.
Pentosidine concentration: sample preparation and analysis
Samples were analyzed in Prof. Hillar Klandorf’s laboratory at West Virginia University, USA. Skin samples were prepared (scraped and minced) as previously described (Cooey et al. 2010). Samples were then processed (delipidation, acid hydrolysis, rehydration) using the standard processing procedure.
Afterwards, 20 µl (standard amount) of samples 1–20 was analyzed using the hydroxyproline (OH-proline) analysis. Upon completion of the analysis, it was discovered that there was very little collagen present in the samples. Samples 21–41 were analyzed using OH-Proline, but 30 µl of sample was used to get a better collagen reading. The spreadsheet equations were updated to account for the volume change for the second half of the samples. Standard curves (Fig. 1 in Supplementary material) for the OH-proline analysis came back indicating that the spectrophotometer was working well.
All samples were then analyzed using reverse-phase high performance liquid chromatography (HPLC) to determine Ps concentration. Standard curve (Fig. 2 in Supplementary material) indicated that the HPLC was producing reliable data. A Ps age prediction curve was then developed for the gull samples.
Telomere length analysis
Telomere was quantified in Prof. Dennis Hasselquist’s laboratory at Lund University, Sweden. DNA was extracted from the blood cells by using phenol/chloroform method and diluted to 1 ng/μl. Telomere length was quantified by using real time quantitative PCR (qPCR) on a Mx3000P q-PCR system (Stratagene) as described by Asghar et al. (2014). We used primers tel (tel 1b 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′ and tel 2b 5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′) described by Criscuolo et al. (2009). For the control of DNA amount in qPCR we used host-specific primers (that amplify an ultra conserved region of vertebrate DNA) sfsr/3 (sfsr/3Fb 5′-ACTAGCCCTTTCAGCGTCATGT-3′and sfsr/3Rb 5′-CATGCTCGGGAACCAAAGG-3′) described by Asghar et al. (2011). Each reaction of 25 μl included 5 μl DNA (1 ng/μl), 12.5 μl Supermix (Platinum SYBR-green q-PCR SuperMix-UDG, Invitrogen), 0.1 μl ROX, 1 μl (10 μM) of the sfsr/3 primers or 0.3 μl (10 μM) of the tel primers and ddH2O. For telomere measurement we ran 30 thermal cycles in qPCR while for the measurement of DNA we ran qPCR for 40 thermal cycles. Thermal cycling condition after incubation for 50 °C for 2 min and 95 °C for 10 min, each cycle was ran at [95 °C for 15 s, (sfsr/3 58 °C for 30 s; tel 60 °C for 30 s) and 72 °C for 30 s]. Each plate contains serially diluted standards (25, 5, 1, 0.2 and 0.04 ng/μl), a reference control (golden sample) and samples all run in duplicate. Tel and sfsr/3 primers were ran on separate plates due to difference in annealing temperatures. We discarded and re-ran qPCR plates that produced standard curves that were outside 85–115 % qPCR efficiency. This method produces high within and across plate repeatability for both sets of primers (Asghar et al. 2014). We then calculated a relative telomere length (T/S ratio) value, by dividing the (plate-adjusted) qPCR value for the telomere length (T) with the (plate-adjusted) qPCR value for the single copy nuclear sequence (S).
Results
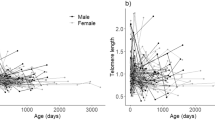
Age of males was associated with the laying date of their partners in a concave manner (Fig. 1a), so that females of middle-aged males laid eggs earlier. Skin pentosidine levels did not correlate with the chronological age of birds (Fig. 1b). Moreover, there was no correlation between skin pentosidine levels and telomere length (Fig. 1c).
Relationships between age and physiological markers among male common gulls. a Age versus laying date: Line: y = 39.9−0.9*x + 0.03*x2, P = 0.006 and 008, for linear and quadratic terms, respectively, n = 47. b Age versus skin pentosidine. c Telomere length versus skin pentosidine. d Age versus telomere length
Telomere length did not show any correlation with chronological age (Fig. 1d). However, the variance in telomere length was 4.6 times higher among the birds older than 13 years (0.14, n = 19) than among the younger breeders (0.03, n = 20; F = 4.1, P = 0.003).
Discussion
Laying date showed a predicted concave relationship with age, indicating that our sample included senescent birds. However, none of our predictions about telomere length or pentosidine were supported. To our knowledge, this is the first study that has failed to detect a positive correlation between skin pentosidine content and chronological age in birds. Previously such positive relationships have been detected in at least nine bird species (Chaney et al. 2003; Fallon et al. 2006; Cooey et al. 2010) with sample sizes ranging from 17 to 450. We had 80 % power to detect a significant positive correlation above r = 0.39 between pentosidine and age. We can thus exclude the possibility that we statistically failed to detect the occurrence of a moderate to strong covariation between skin pentosidine and age. Of note, measurement of hydroxyproline concentration in the patagium was exceptionally low compared to other species, which may have contributed to the anomaly in the measured pentosidine concentrations. Pentosidine levels in our common gulls were in the same range as those recorded in California gulls Larus californicus (Chaney et al. 2003). However, most of other wild birds showed much higher values, ranging up to 150 pmol/mg (Fallon et al. 2006). A mechanistic explanation for the absence of correlation between pentosidine and age in our study may be a low range of variation in pentosidine levels. A functional explanation could be that an association between chronological and physiological age in common gulls is weak. For instance, one might expect that the oldest birds do not comprise a random sample of individuals but appear more viable than the average birds in the population. If viability relates to an ability to slow down or offset the processes that cause accumulation of pentosidine with age, then the correlation between pentosidine levels and age would be weak or absent. Indirect evidence suggests that between-individual differences in pentosidine levels may indeed relate to differences in physiology. For instance, dietary restriction and supplementation with a crosslinking inhibitor suppressed the age-related increase in skin pentosidine in chickens (Iqbal et al. 1999). The physiological differences between genders of birds, however, do not seem to have a influence in pentosidine concentrations, as no significant differences were found between male and female Ruffed Grouse (Bonasa umbellus) of similar ages (Fallon et al. 2006).
Although we had 80 % power to detect a correlation above r = 0.41 between skin pentosidine level and telomere length, no such relationship was found. This finding does not support the view that a common physiological factor (such as oxidative stress) is acting as a major ‘master factor’ imposing the same aging effects throughout the body. Both oxidative and nonoxidative reactions contribute to the chemical modification of tissue proteins by glucose (Iqbal et al. 1999), therefore different physiological mechanisms might cause pentosidine accumulation and telomere attrition. The generality of this outcome, however, remains unknown because we are not aware of any studies that have measured skin pentosidine and telomere length simultaneously. Our results are surprising, given the increased number of studies on birds reporting significant relationships between skin pentosidine levels and age (Chaney et al. 2003; Fallon et al. 2006; Cooey et al. 2010), as well as between telomere length (and shortening) and age (Salomons et al. 2009; Heidinger et al. 2012; Barrett et al. 2013; Bauch et al. 2014). One possible explanation for this lack of covariation between skin pentosidine and telomere length is that these two factors are affected by different physiological processes, and that they therefore in a cross-sectional investigation as in the current study of common gulls, are reflecting different processes of biological aging, thus eroding the relationship between these two aging biomarkers as well as any relationship with chronological age. Also, given the discrepancies between the current study and previous studies in other species indicating associations between age and telomere length and age and pentosidine concentration, it is also possible that these indices of aging are associated with ‘private’ mechanisms of aging—those peculiar to particular evolutionary lineages as opposed to ‘public’ mechanisms—those shared across distantly related evolutionary lineages (Martin et al. 1996).
An increase in variance in telomere length with age (Fig. 1d) in the current study is interesting because it suggests relaxed selection on telomere length among the birds that have passed their prime age of reproduction. Selection hypothesis (Haussmann and Mauck 2008) predicts the opposite: the longest living individuals start with the longest telomeres and variation in telomere length decreases with age due to selective disappearance of individuals with shortest telomeres. Our results, in contrast, demonstrate that birds with a senescent age possessed telomeres of highly variable length. The increase in variation of telomere length with age could reflect either activated telomerase enzyme in the blood stem cells in some birds, individual differences in interstitial telomere gene motifs, or cohort effects.
To conclude, we found no evidence that either skin pentosidine level or telomere length is directly reflecting chronological age in a long-lived seabird exhibiting reproductive senescence. Moreover, these two potent biomarkers of aging showed no covariation. Altogether, these findings suggest that physiological and chronological ages may be largely uncoupled in male common gulls. Furthermore, our findings do not support hypotheses proposing the presence of a common physiological factor (e.g., such as oxidative stress) that would have a general aging-determining effect throughout the body. Instead, our data is compatible with the hypothesis proposing that age-dependent decline of functions is multifactorial and cannot be reduced to one mechanism. Additional research comparing telomere length and pentosidine concentrations to the senescent biomarker beta-galactosidase from additional tissue samples could provide more insight into the best possible age estimation method for long-lived seabirds. Still, our study is based on cross-sectional samples and further longitudinal studies should be very valuable to elucidate how these two aging biomarkers change with age within individuals, allowing us to shed more light on these issues.
References
Asghar M, Hasselquist D, Bensch S (2011) Are chronic avian haemosporidian infections costly in wild birds? J Avian Biol 42:530–537
Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D (2014) Maternal and genetic factors determine early life telomere length. Proc R Soc Lond B 282:20142263
Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S (2015) Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347:436–438
Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS (2013) Telomere length and dynamics predict mortality in a wild longitudinal study. Mol Ecol 22:249–259
Bauch C, Becker PH, Verhulst S (2014) Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol Ecol 23:300–310. doi:10.1111/mec.12602
Brommer JE, Rattiste K (2008) “Hidden” reproductive conflict between mates in a wild bird population. Evolution 62:2326–2333
Brommer J, Rattiste K, Wilson A (2009) The rate of ageing in a long-lived bird is not heritable. Heredity 104:363–370
Chaney RC Jr, Blemings KP, Bonner J, Klandorf H (2003) Pentosidine as a measure of chronological age in wild birds. Auk 120:394–399. doi:10.2307/4090191
Cooey CK, Fallon JA, Avery ML, Anderson JT, Falkenstein EA, Klandorf H (2010) Refinement of biomarker pentosidine methodology for use on aging birds. Hum-Wild Interact 4:304–314
Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG et al (2009) Real-time quantitative PCR assay for measurement of avian telomeres. J Avian Biol 40:342–347
Fallon JA, Cochrane RL, Dorr B, Klandorf H (2006) Interspecies comparison of pentosidine accumulation and its correlation with age in birds. Auk 123:870–876
Haussmann MF, Marchetto NM (2010) Telomeres: linking stress and survival, ecology and evolution. Curr Zool 56:714–727
Haussmann MF, Mauck RA (2008) Telomeres and longevity: testing an evolutionary hypothesis. Mol Biol Evol 25:220–228
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. P Natl Acad Sci USA 109:1743–1748
Holmes D, Martin K (2009) A bird’s-eye view of aging: what’s in it for ornithologists? Auk 126:1–23. doi:10.1525/auk.2009.1109
Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ (2008) Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 44:235–246. doi:10.1016/j.freeradbiomed.2007.10.001
Ilmonen P, Kotrschal A, Penn DJ (2008) Telomere attrition due to infection. PLoS One 3:e2143. doi:10.1371/journal.pone.0002143
Iqbal M, Probert LL, Alhumadi NH, Klandorf H (1999) Protein glycosylation and advanced glycosylated endproducts (AGEs) accumulation: an avian solution? J Gerontol A 54:171–176
Jego M, Lemaitre JF, Bourgoin G, Capron G, Warnant C, Klein F, Gilot-Fromont E, Gaillard JM (2014) Haematological parameters do senesce in the wild: evidence from different populations of a long-lived mammal. J Evol Biol 27:2745–2752
Kilk K, Meitern R, Härmson O, Soomets U, Hõrak P (2014) Assessment of oxidative stress in serum by the d-ROMs test. Free Radic Res 48:883–889. doi:10.3109/10715762.2014.919390
Martin GM, Austad SN, Johnson TE (1996) Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet 13:25–34
Meitern R, Sild E, Kilk K, Porosk R, Hõrak P (2013) On the methodological limitations of detecting oxidative stress: effects of paraquat on measures of oxidative status in greenfinches. J Exp Biol 216:2713–2721. doi:10.1242/jeb.087528
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217:57–66. doi:10.1242/jeb.090043
Monaghan P, Haussmann MF (2006) Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 21:47–53. doi:10.1016/j.tree.2005.11.007
Nussey DH, Froy H, Lemaitre JF, Gaillard JM, Austad SN (2013) Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res Rev 12:214–225
Rattiste K (2004) Reproductive success in presenescent common gulls (Larus canus): the importance of the last year of life. Proc Roy Soc Lond B 271:2059–2064
Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S (2009) Telomere shortening and survival in free-living corvids. Proc Roy Soc Lond B 276:3157–3165
Sepp T, Sild E, Blount JD, Männiste M, Karu U, Hõrak P (2012) Individual consistency and covariation of measures of oxidative status in greenfinches. Phys Biochem Zool 85:299–307
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344. doi:10.1016/S0968-0004(02)02110-2
von Zglinicki T, Martin-Ruiz CM (2005) Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 5:197–203. doi:10.2174/1566524053586545
Acknowledgments
We would like to thank Elizabeth Falkenstein for her technical help with measurement of skin pentosidine, and Ulvi Karu, Richard Meitern and Marju Männiste for their help in the field. The study was financed by the Estonian Ministry of Education and Research (Target-financing Project # 0180004s09, Institutional Research Grant # 2015), the European Union through the European Regional Development Fund (Centre of Excellence Frontiers in Biodiversity Research), Institutional Research Funding (Grants IUT21-1 and IUT34-8) by the Estonian Ministry of Education and Research, the West Virginia Agricultural and Forestry Experimental Station (H608), the Swedish Research Council (to DH), and partly by CAnMove (a Linneaus research excellence center at Lund University funded by the Swedish Research Council and Lund University). The study was conducted under license from the Committee of Animal Experiments at Estonian Ministry of Agriculture (decision # 5, issued on 20 April 2013).
Conflict of interest
We have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10522_2015_9564_MOESM1_ESM.doc
The datasets supporting this article have been uploaded as part of the supplementary material. Supplementary material 1 (DOC 195 kb)
Rights and permissions
About this article
Cite this article
Rattiste, K., Klandorf, H., Urvik, J. et al. Skin pentosidine and telomere length do not covary with age in a long-lived seabird. Biogerontology 16, 435–441 (2015). https://doi.org/10.1007/s10522-015-9564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-015-9564-1