Abstract
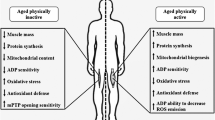
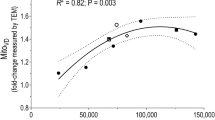
Ageing is associated with several physiological declines to both the cardiovascular (e.g. reduced aerobic capacity) and musculoskeletal system (muscle function and mass). Ageing may also impair the adaptive response of skeletal muscle mitochondria and redox-regulated stress responses to an acute exercise bout, at least in mice and rodents. This is a functionally important phenomenon, since (1) aberrant mitochondrial and redox homeostasis are implicated in the pathophysiology of musculoskeletal ageing and (2) the response to repeated exercise bouts promotes exercise adaptations and some of these adaptations (e.g. improved aerobic capacity and exercise-induced mitochondrial remodelling) offset age-related physiological decline. Exercise-induced mitochondrial remodelling is mediated by upstream signalling events that converge on downstream transcriptional co-factors and factors that orchestrate a co-ordinated nuclear and mitochondrial transcriptional response associated with mitochondrial remodelling. Recent translational human investigations have demonstrated similar exercise-induced mitochondrial signalling responses in older compared with younger skeletal muscle, regardless of training status. This is consistent with data indicating normative mitochondrial remodelling responses to long-term exercise training in the elderly. Thus, human ageing is not accompanied by diminished mitochondrial plasticity to acute and chronic exercise stimuli, at least for the signalling pathways measured to date. Exercise-induced increases in reactive oxygen and nitrogen species promote an acute redox-regulated stress response that manifests as increased heat shock protein and antioxidant enzyme content. In accordance with previous reports in rodents and mice, it appears that sedentary ageing is associated with a severely attenuated exercise-induced redox stress response that might be related to an absent redox signal. In this regard, regular exercise training affords some protection but does not completely override age-related defects. Despite some failed redox-regulated stress responses, it seems mitochondrial responses to exercise training are intact in skeletal muscle with age and this might underpin the protective effect of exercise training on age-related musculoskeletal decline. Whilst further investigation is required, recent data suggest that it is never too late to begin exercise training and that lifelong training provides protection against several age-related declines at both the molecular (e.g. reduced mitochondrial function) and whole-body level (e.g. aerobic capacity).



Similar content being viewed by others
References
Aiken J, Bau E, Cap Z, Lopez M, Wagangat J, McKiernan S (2004) Mitochondrial DNA deletion mutations and sarcopenia. Ann N Y Acad Sci 959:412–423
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M et al (2002) Adaptations of skeletal muscle to exercise: rapid increase in transcriptional co-activator PGC-1. FASEB J 16:1879–1886
Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM et al (2010) Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol 109:449–456
Bartlett JD, Close GL, Drust B, Morton JP (2014) The emerging role of p53 in exercise metabolism. Sports Med 44:303–309
Betik AC, Thomas MM, Wright KJ, Riel CD, Hepple RT (2009) Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Physiol Regul Integr Comp Physiol 297:R744–R755
Booth FW, Laye MJ, Roberts MD (2011) Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol 111:1497–1504
Bori Z, Zhao Z, Kolati E, Fatouros IG, Jamurtas AZ, Dourousdos II et al (2012) The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol 47:417–424
Brigelius-Flohe R, Flohe L (2011) Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 15:2335–2381
Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ (2008) Repeated bouts of aerobic exercise lead to reductions in free radical generation and nuclear kB activation. J Physiol 596:3979–3990
Bua EA, McKeirnan SH, Wanagat J, McKenzie D, Aiken J (2002) Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol 92:2617–2624
Bua EA, Johnson J, Herbst A, Delong B, McKenzie D, Salmat S, Aiken JM (2006) Mitochondrial DNA mutations accumulate to detrimental levels in aged human muscle fibres. Am J Hum Genet 79:469–480
Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL et al (2008) Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586:151–160
Carballal S, Bartesaghi S, Radi R (2014) Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim Biophys Acta 1840:768–780
Close GL, Ashton T, McArdle A, Jackson MJ (2005) Microdialysis studies of extracellular reactive oxygen species production in skeletal muscle: factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Radic Biol Med 39:1460–1467
Close GL, Kayani AC, Ashton T, McArdle A, Jackson MJ (2007) Release of superoxide from skeletal muscle of adult and old mice: an experimental test of the reductive hotspot hypothesis. Aging Cell 6:189–195
Cobley JN, Bartlett JD, Kayani AC, Murray SW, Louhelainen J, Donovan T et al (2012) PGC-1α transcriptional response and mitochondrial adaptation to acute exercise is maintained in skeletal muscle of sedentary elderly males. Biogerontology 13:621–631
Cobley JN, Sakellariou GK, Waldron S, Murray G, Burniston JG, Morton JP et al (2013) Life-long training attenuates residual genotoxic stress in the elderly. Longev Healthspan 2:11
Cobley JN, Sakellariou GK, Owens DJ, Murray S, Waldron S, Gregson W et al (2014) Lifelong training preserves some redox-regulated adaptive responses following an acute exercise stimulus in aged human skeletal muscle. Free Radic Biol Med 70:23–32
Coffey VG, Hawley JA (2007) The molecular basis of training adaptation. Sports Med 37:737–763
Crozier SJ, Kimball SR, Emmert SW, Anthon JC, Jefferson LS (2005) Oral leucine administration stimulates protein Synthesis in rat skeletal muscle. J Nutr 135:376–382
Dai D, Chia YA, Marcinek DJ, Szeto HH, Rabinovtich PS (2014) Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3:6
Debre F, Gomez-Cabrera MC, Nasciemnto AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JAF et al (2012) Age associated low mitochondrial biogenesis may be explained by a lack of response of PGC-1α to exercise training. Age 34:669–679
Demicheli V, Quijano C, Alvarez B, Radi R (2007) Inactivation and nitration of human superoxide dismutase (SOD) by fluxes of nitric oxide and superoxide. Free Radic Biol Med 42:1359–1368
Deschenes MR (2004) Effects of aging on muscle fibre type and size. Sport Med 34:809–824
Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8:870–879
Doherty TJ (2003) Invited review: aging and sarcopenia. J Appl Physiol 111:1717–1727
Drew B, Phaneuf S, Driks A, Selman C, Gredilla R, Lezza A et al (2003) Effects of aging and caloric restriction on mitochondrial energy production in Gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol 284:R474–R480
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184
Evans EM, Racette SB, Petersen LR, Villareal DT, Greiwe JS, Holloszy JO (2005) Aerobic power and insulin action improve in response to endurance exercise training in healthy 77–78 year olds. J Appl Physiol 98:40–45
Faulkner JM, Larkin LM, Claflin DR, Brooks SV (2007) Age-related changes in the structure and function of skeletal muscles. Proc Aust Physiol Soc 38:69–75
Fernadnez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93:884S–890S
Fluck M (2006) Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol 209:2239–2248
Forman HJ, Davies KJA, Ursini F (2014) How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 66:24–35
Frontera WR, Hughes VA, Lutz KJ, Evans WJ (1991) A cross-sectional study of muscle strength and mass in 45–78 year old men and women. J Appl Physiol 71:644–650
Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP et al (2011) Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 60:2051–2060
Gibala M, Little JP, MacDonald MJ, Hawley JA (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590:1077–1084
Godin R, Ascah A, Daussin FN (2010) Intensity-dependent activation of intracellular signaling pathways in skeletal muscle: role of fibre type recruitment during exercise. J Physiol 588:4073–4074
Gousillou G, Sgarioto N, Kapchinksy S, Purves-Smith F, Norris B, Pion CH et al (2014) Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 28:1621–1633
Gutteridge JMC, Halliwell B (2010) Antioxidants: molecules, medicines, and myths. Biochem Biophys Res Commun 393:561–564
Halliwell B, Gutteridge JMG (2007) Free Radicals in Biology & Medicine, 4th edn. Oxford University Press, Oxford
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Heath GW, Hagberg JM, Ehsani AA, Holloszy JO (1981) A physiological comparison of young and older endurance athletes. J Appl Physiol 51:634–640
Hiona A, Leeuwenburgh C (2008) The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol 43:23–33
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56:831–838
Holmstrom KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15:411–421
Hood DA (2001) Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90:1137–1157
Hood DA (2009) Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 34:465–472
Irrcher R, Ljubicic V, Hood DA (2009) Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296:C116–C123
Iversen N, Krsutrup P, Rasmussen HN, Rasmussen UF, Saltin B, Pilegaard H (2011) Mitochondrial biogenesis and angiogenesis in muscle of the elderly. Exp Gerontol 46:670–678
Jackson MJ (2009) Redox regulation of adaptive responses of skeletal muscle to contractile activity. Free Radic Biol Med 47:1267–1275
Jackson MJ, McArdle A (2011) Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589:2139–2145
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA 104:12017–12022
Jang YC, Van Remmen H (2009) The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol 44:256–260
Jang YC, Van Remmen H (2011) Age-associated alterations of the neuromuscular junction. Exp Gerontol 46:193–198
Janssen I, Shepard P, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52:80–85
Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T et al (2008) Redox-based regulation of signal transduction: principles: pitfalls, and promises. Free Radic Biol Med 45:1–17
Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J (2004) Acute exercise activates nuclear factor (NF)-KB signalling pathway in rat skeletal muscle. FASEB J 18:1499–1506
Ji LL, Gomez-Cabrera MC, Vina J (2006) Exercise and hormesis: activation of cellular antioxidant signalling pathway. Ann NY Acad Sci 1067:425–435
Joesph AM, Adhihttey PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguen LM et al (2012) The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low functioning elderly individuals. Aging Cell 11:801–809
Kalyanaraman B, Usmar V, Davies KJA, Dennery PA, Forman RJ, Grisham MB et al (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6
Kayani AC, Morton JP, McArdle A (2008) The exercise-induced stress response in skeletal muscle: failure during aging. Appl Physiol Nutr Metab 33:1033–1041
Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ (2001) Time course of adaptive responses of human skeletal muscle to oxidative stress induced by non-damaging exercise. J Appl Physiol 90:1031–1036
Kholodenko B, Hancock JF, Kolch W (2010) Cell signalling ballet in space and time. Nat Rev Mol Cell Biol 11:414–426
Koltai E, Hart N, Taylor AW, Goto S, Nyo JK, Davies KJ et al (2012) Age-associated declines in mitochondrial biogenesis are minimised by exercise training. Am J Physiol Regul Integr Comp Physiol 303:R127–R134
Konopka AR, Nair SK (2013) Mitochondrial and skeletal muscle health with advancing age. Mol Cell Endocrinol 379:19–29
Konopka AR, Suer MK, Wolff CA, Harber MP (2014) Markers of human skeletal muscle mitochondrial biogenesis and quality control. Effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci 69:371–378
Lanza IR, Nair KS (2010) Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol 199:529–547
Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ et al (2008) Endurance exercise as a countermeasure for aging. Diabetes 57:2933–2942
Leick L, Wojtaszewski JFP, Johansen ST, Kiilerich K, Gomes G, Hellsten Y et al (2008) PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294:E463–E474
Lexell J, Taylor CC, Sjostrum M (1988) What is the cause of ageing atrophy? Total number, size and proportion of different fibre types studied in whole vastus lateralis muscle from 15 to 83 year old men. J Neurol Sci 84:275–294
Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ (2010a) Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298:R912–R917
Little JP, Safdar A, Wilkin GP, Tranopolsky MA, Gibala MJ (2010b) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588:1011–1022
Ljubicic V, Hood DA (2009a) Diminished contraction-induced intracellular signalling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell 8:394–404
Ljubicic V, Hood DA (2009b) Specific attenuation of protein kinase phosphorylation in muscle with a high mitochondrial content. Am J Physiol Endocrinol Metab 297:E749–E758
Ljubicic V, Joesph A, Adhihetty PJ, Huang JH, Saleem A, Uguccioni G et al (2009) Molecular basis for an attenuated mitochondrial plasticity in aged skeletal muscle. Aging 9:818–830
Ljubicic V, Joseph AM, Saleem A, Uguccioni G, Collu-Marchese M, Lai RY et al (2010) Transcriptional and post-transcriptional regulation of mitochondrial biogenesis in skeletal muscle: effects of exercise and aging. Biochim Biophys Acta 1800:223–234
Lopez-Lluch G, Irusta PM, Navas P, de Cabo R (2008) Mitochondrial biogenesis and healthy aging. Exp Gerontol 43:813–819
Mahoney DJ, Praise G, Melov S, Safdar A, Tarnopolsky MA (2005) Analysis of global mRNA expression from human skeletal muscle during recovery from endurance exercise. FASEB J 19:1498–1500
Margaritelis NV, Kyparos A, Paschalis V, Theodorou AA, Panayiotou G, Zafeiridis A et al (2014) Reductive stress after exercise: the issue of redox individuality. Redox Biol 2:520–528
Martinez-Ruiz A, Cadenas S, Lamas S (2011) Nitric oxide signalling: classical, less classical and nonclassical mechanisms. Free Radic Biol Med 51:17–29
McArdle A, Patwell D, Vasilaki A, Griffiths RD, Jackson MJ (2001) Contractile-activity induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280:C621–C627
McDonagh B, Sakellariou GK, Smith N, Brownridge P, Jackson MJ (2014) Differential cysteine labelling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J Prot Res. doi:10.1021/pr5006394
McGlory C, White A, Treins C, Drust B, Close GL, MacLaren DPM et al (2014) Application of the [gamma-P-32] ATP kinase assay to study anabolic signaling in human skeletal muscle. J Appl Physiol 116:504–513
McKenzie D, Bua E, McKiernan S, Cao Z, Wanagat J, Aiken JM (2002) Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Euro J Biochem 269:2010–2015
Melov A, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A (2007) Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2:e465
Menshikova EV, Ritov VB, Fairfull L, Ferrel RE, Kelley DE, Goodpaster BH (2006) Effects of exercise on mitochondrial content and function in aging human muscle. J Gerontol 61:534–540
Miller BF, Hamilton KL (2012) A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab 302:E496–E499
Morton JP, MacLaren DPM, Cable NT, Bongers T, Griffiths RD, Campbell IT et al (2006) Time-course and differential responses of the major heat shock protein families in human skeletal muscle following acute non-damaging treadmill exercise. J Appl Physiol 101:176–182
Morton JP, Kayani AC, McArdle A, Drust B (2009) The exercise-induced stress response of skeletal muscle with specific emphasis on humans. Sports Med 39:643–662
Murphy MP, Holmgren A, Larsson N, Halliwell B, Chang CJ, Kalyanaraman B et al (2011) Unravelling the biological roles of reactive oxygen species. Cell Metab 13:361–366
Nordsborg NB, Lundby C, Leick L, Pilegaard H (2010) Relative workload determine exercise-induced increases in PGC-1alpaha mRNA. Med Sci Sports Exerc 42:1477–1484
Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP (2012) Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590:5361–5370
Nyberg M, Mortensen SP, Cabo H, Gomez-Cabrera MC, Vina J, Hellensten Y (2014) Role of sedentary aging and lifelong physical activity on exchange of glutathione across exercising human skeletal muscle. Free Radic Biol Med 73:166–173
Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralink JM et al (2006) Effects of a physical activity intervention on measures of physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci 61:1157–1165
Palomero J, Pye D, Kabayo T, Spiller DG, Jackson MJ (2008) In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibres by real-time fluorescence microscopy. Antioxid Redox Signal 10:1463–1474
Palomero J, Vasilaki A, Pye D, McArdle A, Jackson MJ (2013) Aging increases the oxidation of dichlorohydrofluorescein in single isolated skeletal muscle fibres at rest, but not during contractions. Am J Physiol Regul Integr Comp Physiol 305:R351–R358
Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M et al (2002) Muscle function in elite master weightlifters. Med Sci Sports Exerc 34:1199–1206
Pearson T, McArdle A, Jackson MJ (2014) Nitric oxide availability is increased in contracting skeletal muscle from aged mice, but does not differentially decrease muscle superoxide. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2014.10.505i
Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588:4795–4810
Peterson CM, Johannsen DL, Ravussin E (2012) Skeletal muscle mitochondria and aging: a review. J Aging Res 2012:194821
Philp A, Chen A, Lan D, Meyer GA, Murphy A, Knapp AE et al (2011) Sirtuin 1 (SIRT1) deactylase activity is not required for mitochondrial biogenesis or peroxisome proliferator activate receptor-γ co-activator-1α (PGC-1α) deactylation following exercise. J Biol Chem 286:30561–30570
Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL et al (2010) Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permealised fibres. Aging Cell 6:1032–1046
Picard M, Taivassalo T, Gouspillou G, Hepple RT (2011a) Mitochondria: isolation, structure and function. J Physiol 589:4421–4431
Picard M, Ritchie D, Thomas MM, Wright KJ, Hepple RT (2011b) Alterations in intrinsic mitochondrial function with aging are fibre type-specific and do not explain differential atrophy between muscles. Aging Cell 10:1047–1055
Picard M, Shirihai OS, Gentil BJ, Burelle Y (2013) Mitochondrial morphology transitions and functions: implications for retrograde signalling. Am J Physiol Regul Integr Comp Physiol 304:R393–R406
Pilegaard H, Saltin B, Neufer DP (2003) Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546:851–858
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Pye D, Palomero J, Kabayo T, Jackson MJ (2007) Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol 581:309–318
Radak Z, Bori Z, Kolati E, Fatourous GI, Jamaturas AZ, Douroudos II et al (2011) Age-dependent changes in 8-oxoguanine-DNA glyocloase activity are modulated by the adaptive response to physical exercise in human muscle. Free Radic Biol Med 51:417–423
Rennie MJ, Selby A, Atherton P, Smith K, Glover EL, Phillips SM (2010) Facts noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scand J Med Sci Sport 20:5–9
Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ et al (2007) Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5:151–156
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 11:872–884
Rodgers JT, Lerin C, Haas W, Gygy SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118
Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z (2012) PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE 7:e41817
Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C et al (2003) Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-γ coactivator-1α in skeletal muscle. Diabetes 52:2874–28812
Safdar A, DeBeer J, Tarnopolsky MA (2010a) Dysfunctional Nrf2-Keap1 redox signalling in skeletal muscle of the sedentary old. Free Radic Biol Med 49:1487–1493
Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, deBeer J, Tranopolsky MA (2010b) Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary adults. PLoS ONE 5:e10788
Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA (2011) Exercise increases mitochondrial PGC-1α content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 286:10605–10617
Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A et al (2013) Studies of mitochondrial and non-mitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase (s) in the increase skeletal muscle superoxide generation that occurs following contractile activity. Antiox Redox Signal 18:603–621
Sakellariou GK, Jackson MJ, Vasilaki A (2014) Redefining the major contributors to superoxide production in contracting skeletal muscle. Role of NAD(P)H oxidases. Free Radic Res 48:12–29
Saleem A, Hood DA (2013) Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. J Physiol 591:3625–3636
Salmon AB, Richardson A, Perez VI (2010) Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 48:642–655
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH et al (2006) PGC-1alpha protects form atrophy by suppressing FOXO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Scarpulla RC (2008) Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88:611–638
Short KR, Vittone JL, Brigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM et al (2003) Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623
Sies H (2014) Role of metabolic H2O2 generation: redox signalling and oxidative stress. J Biol Chem. doi:10.1074/jbc.R113.544635
Stuart JA, Maddalena LA, Merilovich M, Robb EL (2014) A midlife crisis for the mitochondrial free radical theory of aging. Longev Healthspan. 3:4
Tanaka H, Seals DR (2008) Endurance exercise and performance in masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol 586:55–63
Tanaka H, DeSouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR (1997) Greater decline in maximal aerobic capacity with age in physically active vs sedentary healthy women. J Appl Physiol 83:1947–1953
Timmons JA (2011) Variability in training-induced skeletal muscle adaptation. J Appl Physiol 110:846–853
Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T (1996) Aging among elite distance runners: a 22 year longitudinal study. J Appl Physiol 80:285–290
Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W et al (2013) New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol 114:3–10
Valls MRB, Wilkinson DJ, Narici M, Smith K, Phillips BE et al (2014) Protein carbonylation and heat shock proteins in human skeletal muscle: relationships to age and sarcopenia. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glu007
Vasilaki A, Jackson MJ, McArdle A (2002) Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve 25:902–905
Vasilaki A, Iwanejko I, McArdle F, Broome CS, Jackson MJ, McArdle A (2003) Skeletal muscle of aged male mice fail to adapt following contractile activity. Biochem Soc Trans 31:455–456
Vasilaki A, McArdle F, Iwanejko LM, McArdle A (2006a) Adaptive response of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev 127:830–839
Vasilaki A, Mansouri A, Van Remmen H, Van Der Meulen JH, Larkin L, Richardson AG et al (2006b) Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5:109–117
Wenz T (2013) Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion 13:134–142
Wenz T, Rosse SG, Rotundo RL, Spiegelman BM, Moraes CT (2009) Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease. Proc Natl Acad Sci USA 106:20405–20410
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884
Winterbourn CC (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4:278–286
Winterbourn CC (2014a) Are free radicals involved in thiol-based redox signalling? Free Radic Biol Med. doi:10.1016/j.freeradbiolmed.2014.08.017i
Winterbourn CC (2014b) The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 1840:730–738
Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO (2007) Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem 282:194–199
Acknowledgments
Age UK are thanked for generous financial support. JNC and PRM would like to acknowledge financial support provided by the Carnegie Trust and Abertay University.
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cobley, J.N., Moult, P.R., Burniston, J.G. et al. Exercise improves mitochondrial and redox-regulated stress responses in the elderly: better late than never!. Biogerontology 16, 249–264 (2015). https://doi.org/10.1007/s10522-014-9546-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-014-9546-8