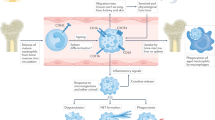
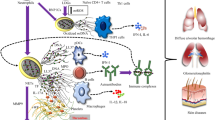
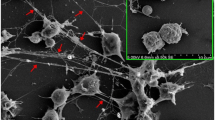
Abstract
Autoimmune diseases are pathological conditions that result from the misidentification of self-antigens in immune system, leading to host tissue damage and destruction. These diseases can affect different organs and systems, including the blood, joints, skin, and muscles. Despite the significant progress made in comprehending the underlying pathogenesis, the complete mechanism of autoimmune disease is still not entirely understood. In autoimmune diseases, the innate immunocytes are not functioning properly: they are either abnormally activated or physically disabled. As a vital member of innate immunocyte, neutrophils and their modes of death are influenced by the microenvironment of different autoimmune diseases due to their short lifespan and diverse death modes. Related to neutrophil death pathways, apoptosis is the most frequent cell death form of neutrophil non-lytic morphology, delayed or aberrant apoptosis may contribute to the development anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV). In addition, NETosis, necroptosis and pyroptosis which are parts of lytic morphology exacerbate disease progression through various mechanisms in autoimmune diseases. This review aims to summarize recent advancements in understanding neutrophil death modes in various autoimmune diseases and provide insights into the development of novel therapeutic approaches for autoimmune diseases.


Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ANCA:
-
Anti-neutrophil cytoplasmic antibodies
- RA:
-
Rheumatoid arthritis
- SS:
-
Sjogren's syndrome
- UC:
-
Ulcerative colitis
- MS:
-
Multiple sclerosis
- NE:
-
Neutrophil elastase
- GM-CSF:
-
Granulocyte colony-stimulating factor
- NETs:
-
Neutrophil extracellular traps
- DAMPs:
-
Damage-associated molecular patterns
- TNF-α:
-
Tumor necrosis factor-α
- GPA:
-
Granulomatosis with polyangiitis
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- LRP:
-
Lipoprotein receptor-related protein
- HA:
-
Hyaluronic acid
- PD-L1:
-
Programmed cell death-ligand 1
- PBMC:
-
Peripheral blood mononuclear cell
- ANA:
-
Antinuclear antibodies
- HNP:
-
Human neutrophil peptide
- TLR9:
-
Toll-like receptor 9
- IC:
-
Immune complexes
- MPA:
-
Microscopic poly-vasculitis
- HMGB1:
-
High mobility group protein 1
- ACPAs:
-
Anti-citrullinated peptide antibodies
- SF:
-
Synovial fluid
- TEN:
-
Toxic epidermal necrolysis
- LPMC:
-
Lamina propria mononuclear cells
- TNBS:
-
2,4,6-Trinitrobenzene sulfonic acid
- pfCD:
-
Perianal fistulising Crohn's disease
- μPs:
-
Microparticles
- aPL:
-
An-tiphospholipid antibody
- FAP-a:
-
Fibroblast-activated protein-a
- MRSA:
-
Methicillin-resistant staphylococcus aureus
- AS:
-
Ankylosing spondylitis
- AIH:
-
Autoimmune-mediated hepatitis
- HSCs:
-
Hematopoietic stem cells
- LMWH:
-
Low molecular weight heparins
- PMN:
-
Polymorphonuclear neutrophils
- EGPA:
-
Eosinophilic granuloma with poly-vasculitis
- Hif-1α:
-
Hypoxia-inducible factor-1α
- LAMP-2:
-
Lysosomal membrane protein-2
- RNP IC:
-
Immunocomplexes containing ribonucleoproteins
- TIM-3:
-
T cell immunoglobulin and mucin domain-containing protein 3
- MCPIP-1:
-
Monocyte chemotactic protein-inducible protein-1
- AAV:
-
ANCA-associated vasculitis
- SLE:
-
Systemic lupus erythematosus
- SSC:
-
Systemic sclerosis
- CD:
-
Crohn's disease
- MPO:
-
Myeloperoxidase
- ROS:
-
Reactive oxygen species
- Cyt-C:
-
Cytochrome C
- CTL:
-
Cytotoxic T lymphocytes
- NK:
-
Natural killer cells
- PR3:
-
Proteinase 3
- CRT:
-
Calreticulin
- APS:
-
Antiphospholipid syndrome
- ECM:
-
Extracellular cell matrix
- IFN-I:
-
Type I interferon
- MIF:
-
Migration inhibitory factor
- PAD4:
-
Peptidylarginine deiminase 4
- LDG:
-
Low-density granulocytes
- pDC:
-
Plasmacytoid dendritic cells
- MMP-9:
-
Matrix metalloproteinase-9
- EC:
-
Endothelial cells
- mDCs:
-
Myeloid dendritic cells
- TF:
-
Tissue factor
- IL-17A:
-
Interleukin-17A
- SJS:
-
Stevens Johnson Syndrome
- FPR1:
-
Rormyl peptide receptor 1
- HOCl:
-
Oxidant hypochlorous acid
- RRMS:
-
Relapsing–remitting MS
- ANETA:
-
Anti-NET antibodies
- PSGL-1:
-
P-selectin
- aCL:
-
An-ticardiolipin antibody
- MSU:
-
Monosodium urate
- PM:
-
Plasma membrane
- AOSD:
-
Adult Still's disease (AOSD)
- Atg:
-
Autophagy related gene
- CQ:
-
Chloroquine
- MA:
-
3-Methyl-adenine
- ET-1:
-
Endothelin-1
References
Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J Intern Med 278:369–395. https://doi.org/10.1111/joim.12395
Lesage S, Goodnow CC (2001) Organ-specific autoimmune disease: a deficiency of tolerogenic stimulation. J Exp Med 194:F31-36. https://doi.org/10.1084/jem.194.5.f31
Wahren-Herlenius M, Dörner T (2013) Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 382:819–831. https://doi.org/10.1016/s0140-6736(13)60954-x
Ma WT, Gao F, Gu K, Chen DK (2019) The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front Immunol 10:1140. https://doi.org/10.3389/fimmu.2019.01140
Gianchecchi E, Delfino DV, Fierabracci A (2018) NK cells in autoimmune diseases: linking innate and adaptive immune responses. Autoimmun Rev 17:142–154. https://doi.org/10.1016/j.autrev.2017.11.018
Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A (2021) The neutrophil. Immunity 54:1377–1391. https://doi.org/10.1016/j.immuni.2021.06.006
de Oliveira S, Rosowski EE, Huttenlocher A (2016) Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16:378–391. https://doi.org/10.1038/nri.2016.49
Liew PX, Kubes P (2019) The neutrophil’s role during health and disease. Physiol Rev 99:1223–1248. https://doi.org/10.1152/physrev.00012.2018
Németh T, Sperandio M, Mócsai A (2020) Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov 19:253–275. https://doi.org/10.1038/s41573-019-0054-z
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. https://doi.org/10.1038/nri3399
Vicar T, Raudenska M, Gumulec J, Balvan J (2020) The quantitative-phase dynamics of apoptosis and lytic cell death. Sci Rep 10:1566. https://doi.org/10.1038/s41598-020-58474-w
Jorgensen I, Rayamajhi M, Miao EA (2017) Programmed cell death as a defence against infection. Nat Rev Immunol 17:151–164. https://doi.org/10.1038/nri.2016.147
Lawrence SM, Corriden R, Nizet V (2020) How neutrophils meet their end. Trends Immunol 41:531–544. https://doi.org/10.1016/j.it.2020.03.008
Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, Lemnitzer P, Ortega-Gómez A, Chevre R, Marschner J, Schumski A, Winter C, Perez-Olivares L, Pan C, Paulin N, Schoufour T, Hartwig H, González-Ramos S, Kamp F, Megens RTA, Mowen KA, Gunzer M, Maegdefessel L, Hackeng T, Lutgens E, Daemen M, von Blume J, Anders HJ, Nikolaev VO, Pellequer JL, Weber C, Hidalgo A, Nicolaes GAF, Wong GCL, Soehnlein O (2019) Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569:236–240. https://doi.org/10.1038/s41586-019-1167-6
Obeng E (2021) Apoptosis (programmed cell death) and its signals - a review. Braz J Biol 81:1133–1143. https://doi.org/10.1590/1519-6984.228437
Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489–517. https://doi.org/10.1146/annurev-immunol-042617-053010
Zhang Q, Liu J, Zhang M, Wei S, Li R, Gao Y, Peng W, Wu C (2019) Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules. https://doi.org/10.3390/biom9120795
Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG (2010) Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun 2:216–227. https://doi.org/10.1159/000284367
Millet A, Martin KR, Bonnefoy F, Saas P, Mocek J, Alkan M, Terrier B, Kerstein A, Tamassia N, Satyanarayanan SK, Ariel A, Ribeil JA, Guillevin L, Cassatella MA, Mueller A, Thieblemont N, Lamprecht P, Mouthon L, Perruche S, Witko-Sarsat V (2015) Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J Clin Invest 125:4107–4121. https://doi.org/10.1172/jci78182
Jerke U, Eulenberg-Gustavus C, Rousselle A, Nicklin P, Kreideweiss S, Grundl MA, Eickholz P, Nickles K, Schreiber A, Korkmaz B, Kettritz R (2022) Targeting cathepsin c in PR3-ANCA vasculitis. J Am Soc Nephrol. https://doi.org/10.1681/asn.2021081112
Everts-Graber J, Martin KR, Thieblemont N, Mocek J, Roccabianca A, Chafey P, Le Gall M, Tacnet-Delorme P, Reutelingsperger CP, Naccache JM, Bonnotte B, Karras A, Puéchal X, Guillevin L, Terrier B, Frachet P, Perretti M, Mouthon L, Witko-Sarsat V (2019) Proteomic analysis of neutrophils in ANCA-associated vasculitis reveals a dysregulation in proteinase 3-associated proteins such as annexin-A1 involved in apoptotic cell clearance. Kidney Int 96:397–408. https://doi.org/10.1016/j.kint.2019.02.017
Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, Lyons PA, Merkel PA, Savage COS, Specks U, Kain R (2020) ANCA-associated vasculitis. Nat Rev Dis Primers 6:71. https://doi.org/10.1038/s41572-020-0204-y
Gabillet J, Millet A, Pederzoli-Ribeil M, Tacnet-Delorme P, Guillevin L, Mouthon L, Frachet P, Witko-Sarsat V (2012) Proteinase 3, the autoantigen in granulomatosis with polyangiitis, associates with calreticulin on apoptotic neutrophils, impairs macrophage phagocytosis, and promotes inflammation. J Immunol 189:2574–2583. https://doi.org/10.4049/jimmunol.1200600
Müller A, Krause B, Kerstein-Stähle A, Comdühr S, Klapa S, Ullrich S, Holl-Ulrich K, Lamprecht P (2021) Granulomatous inflammation in ANCA-associated vasculitis. Int J Mol Sci. https://doi.org/10.3390/ijms22126474
Greenlee-Wacker MC (2016) Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev 273:357–370. https://doi.org/10.1111/imr.12453
van Rossum AP, Limburg PC, Kallenberg CG (2005) Activation, apoptosis, and clearance of neutrophils in Wegener’s granulomatosis. Ann N Y Acad Sci 1051:1–11. https://doi.org/10.1196/annals.1361.041
Moosig F, Csernok E, Kumanovics G, Gross WL (2000) Opsonization of apoptotic neutrophils by anti-neutrophil cytoplasmic antibodies (ANCA) leads to enhanced uptake by macrophages and increased release of tumour necrosis factor-alpha (TNF-alpha). Clin Exp Immunol 122:499–503. https://doi.org/10.1046/j.1365-2249.2000.01410.x
Casciola-Rosen LA, Anhalt G, Rosen A (1994) Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 179:1317–1330. https://doi.org/10.1084/jem.179.4.1317
Sandin C, Eriksson P, Segelmark M, Skogh T, Kastbom A (2016) IgA- and SIgA anti-PR3 antibodies in serum versus organ involvement and disease activity in PR3-ANCA-associated vasculitis. Clin Exp Immunol 184:208–215. https://doi.org/10.1111/cei.12769
Defendi F, Thielens NM, Clavarino G, Cesbron JY, Dumestre-Pérard C (2020) The immunopathology of complement proteins and innate immunity in autoimmune disease. Clin Rev Allergy Immunol 58:229–251. https://doi.org/10.1007/s12016-019-08774-5
Donnelly S, Roake W, Brown S, Young P, Naik H, Wordsworth P, Isenberg DA, Reid KB, Eggleton P (2006) Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum 54:1543–1556. https://doi.org/10.1002/art.21783
Tacnet-Delorme P, Gabillet J, Chatfield S, Thieblemont N, Frachet P, Witko-Sarsat V (2018) Proteinase 3 interferes with C1q-mediated clearance of apoptotic cells. Front Immunol 9:818. https://doi.org/10.3389/fimmu.2018.00818
Trouw LA, Daha N, Kurreeman FA, Böhringer S, Goulielmos GN, Westra HJ, Zhernakova A, Franke L, Stahl EA, Levarht EW, Stoeken-Rijsbergen G, Verduijn W, Roos A, Li Y, Houwing-Duistermaat JJ, Huizinga TW, Toes RE (2013) Genetic variants in the region of the C1q genes are associated with rheumatoid arthritis. Clin Exp Immunol 173:76–83. https://doi.org/10.1111/cei.12097
Wight TN, Kang I, Evanko SP, Harten IA, Chang MY, Pearce OMT, Allen CE, Frevert CW (2020) Versican-A critical extracellular matrix regulator of immunity and inflammation. Front Immunol 11:512. https://doi.org/10.3389/fimmu.2020.00512
Gee K, Kryworuchko M, Kumar A (2004) Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 52:13–26
Nagy N, Kuipers HF, Marshall PL, Wang E, Kaber G, Bollyky PL (2019) Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol 78–79:292–313. https://doi.org/10.1016/j.matbio.2018.03.022
Wittig B, Seiter S, Schmidt DS, Zuber M, Neurath M, Zöller M (1999) CD44 variant isoforms on blood leukocytes in chronic inflammatory bowel disease and other systemic autoimmune diseases. Lab Invest 79:747–759
Kitano A, Oshitani N, Matsumoto T, Kobayashi K (1996) CD44 variants in ulcerative colitis and Crohn’s disease. Lancet 348:266–267. https://doi.org/10.1016/s0140-6736(05)65573-0
Takazoe K, Tesch GH, Hill PA, Hurst LA, Jun Z, Lan HY, Atkins RC, Nikolic-Paterson DJ (2000) CD44-mediated neutrophil apoptosis in the rat. Kidney Int 58:1920–1930. https://doi.org/10.1111/j.1523-1755.2000.00364.x
Cairns AP, Crockard AD, McConnell JR, Courtney PA, Bell AL (2001) Reduced expression of CD44 on monocytes and neutrophils in systemic lupus erythematosus: relations with apoptotic neutrophils and disease activity. Ann Rheum Dis 60:950–955. https://doi.org/10.1136/ard.60.10.950
Lessard CJ, Adrianto I, Kelly JA, Kaufman KM, Grundahl KM, Adler A, Williams AH, Gallant CJ, Anaya JM, Bae SC, Boackle SA, Brown EE, Chang DM, Criswell LA, Edberg JC, Freedman BI, Gregersen PK, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martin J, Merrill JT, Niewold TB, Park SY, Petri MA, Pons-Estel BA, Ramsey-Goldman R, Reveille JD, Song YW, Stevens AM, Tsao BP, Vila LM, Vyse TJ, Yu CY, Guthridge JM, Bruner GR, Langefeld CD, Montgomery C, Harley JB, Scofield RH, Gaffney PM, Moser KL (2011) Identification of a systemic lupus erythematosus susceptibility locus at 11p13 between PDHX and CD44 in a multiethnic study. Am J Hum Genet 88:83–91. https://doi.org/10.1016/j.ajhg.2010.11.014
Karmakar U, Chu JY, Sundaram K, Astier AL, Garside H, Hansen CG, Dransfield I, Vermeren S (2021) Immune complex-induced apoptosis and concurrent immune complex clearance are anti-inflammatory neutrophil functions. Cell Death Dis 12:296. https://doi.org/10.1038/s41419-021-03528-8
Zhu M, Yuan K, Lu Q, Zhu Q, Zhang S, Li X, Zhao L, Wang H, Luo G, Wang T, Huang G, Xu A (2019) Emodin ameliorates rheumatoid arthritis by promoting neutrophil apoptosis and inhibiting neutrophil extracellular trap formation. Mol Immunol 112:188–197. https://doi.org/10.1016/j.molimm.2019.05.010
Andrews LP, Yano H, Vignali DAA (2019) Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol 20:1425–1434. https://doi.org/10.1038/s41590-019-0512-0
Zamani MR, Aslani S, Salmaninejad A, Javan MR, Rezaei N (2016) PD-1/PD-L and autoimmunity: a growing relationship. Cell Immunol 310:27–41. https://doi.org/10.1016/j.cellimm.2016.09.009
Wang JF, Wang YP, Xie J, Zhao ZZ, Gupta S, Guo Y, Jia SH, Parodo J, Marshall JC, Deng XM (2021) Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood 138:806–810. https://doi.org/10.1182/blood.2020009417
Tanaka H, Arima Y, Kamimura D, Tanaka Y, Takahashi N, Uehata T, Maeda K, Satoh T, Murakami M, Akira S (2019) Phosphorylation-dependent Regnase-1 release from endoplasmic reticulum is critical in IL-17 response. J Exp Med 216:1431–1449. https://doi.org/10.1084/jem.20181078
Dobosz E, Wadowska M, Kaminska M, Wilamowski M, Honarpisheh M, Bryzek D, Potempa J, Jura J, Lech M, Koziel J (2021) MCPIP-1 restricts inflammation via promoting apoptosis of neutrophils. Front Immunol 12:627922. https://doi.org/10.3389/fimmu.2021.627922
Qu B, Cao J, Zhang F, Cui H, Teng J, Li J, Liu Z, Morehouse C, Jallal B, Tang Y, Guo Q, Yao Y, Shen N (2015) Type I interferon inhibition of microRNA-146a maturation through Up-regulation of monocyte chemotactic protein-induced protein 1 in systemic lupus erythematosus. Arthritis Rheumatol 67:3209–3218. https://doi.org/10.1002/art.39398
Schindler L, Zwissler L, Krammer C, Hendgen-Cotta U, Rassaf T, Hampton MB, Dickerhof N, Bernhagen J (2021) Macrophage migration inhibitory factor inhibits neutrophil apoptosis by inducing cytokine release from mononuclear cells. J Leukoc Biol 110:893–905. https://doi.org/10.1002/jlb.3a0420-242rrr
El Kebir D, József L, Khreiss T, Filep JG (2006) Inhibition of K+ efflux prevents mitochondrial dysfunction, and suppresses caspase-3-, apoptosis-inducing factor-, and endonuclease G-mediated constitutive apoptosis in human neutrophils. Cell Signal 18:2302–2313. https://doi.org/10.1016/j.cellsig.2006.05.013
Ottonello L, Cutolo M, Frumento G, Arduino N, Bertolotto M, Mancini M, Sottofattori E, Dallegri F (2002) Synovial fluid from patients with rheumatoid arthritis inhibits neutrophil apoptosis: role of adenosine and proinflammatory cytokines. Rheumatology (Oxford) 41:1249–1260. https://doi.org/10.1093/rheumatology/41.11.1249
Papayannopoulos V (2018) Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18:134–147. https://doi.org/10.1038/nri.2017.105
Sørensen OE, Borregaard N (2016) Neutrophil extracellular traps - the dark side of neutrophils. J Clin Investig 126:1612–1620. https://doi.org/10.1172/jci84538
Yipp BG, Kubes P (2013) NETosis: how vital is it? Blood 122:2784–2794. https://doi.org/10.1182/blood-2013-04-457671
Jariwala MP, Laxer RM (2021) NETosis in rheumatic diseases. Current Rheumatol Rep 23:9. https://doi.org/10.1007/s11926-020-00977-6
Lou H, Wojciak-Stothard B, Ruseva MM, Cook HT, Kelleher P, Pickering MC, Mongkolsapaya J, Screaton GR, Xu XN (2020) Autoantibody-dependent amplification of inflammation in SLE. Cell Death Dis 11:729. https://doi.org/10.1038/s41419-020-02928-6
Bai Y, Tong Y, Liu Y, Hu H (2018) Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin Exp Immunol 191:1–10. https://doi.org/10.1111/cei.13041
Knight JS, Subramanian V, O’Dell AA, Yalavarthi S, Zhao W, Smith CK, Hodgin JB, Thompson PR, Kaplan MJ (2015) Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis 74:2199–2206. https://doi.org/10.1136/annrheumdis-2014-205365
Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ (2016) Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 22:146–153. https://doi.org/10.1038/nm.4027
Dörner T (2012) SLE in 2011: deciphering the role of NETs and networks in SLE. Nat Rev Rheumatol 8:68–70. https://doi.org/10.1038/nrrheum.2011.200
Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V (2011) Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3:73ra20. https://doi.org/10.1126/scitranslmed.3001201
Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, Dummer R, Simon HU, Yousefi S (2004) Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem 279:44123–44132. https://doi.org/10.1074/jbc.M405883200
Pieterse E, Hofstra J, Berden J, Herrmann M, Dieker J, van der Vlag J (2015) Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clin Exp Immunol 179:68–74. https://doi.org/10.1111/cei.12359
Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ (2015) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 74:1417–1424. https://doi.org/10.1136/annrheumdis-2013-204837
Blanco LP, Wang X, Carlucci PM, Torres-Ruiz JJ, Romo-Tena J, Sun HW, Hafner M, Kaplan MJ (2021) RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol 73:2282–2292. https://doi.org/10.1002/art.41796
Yang B, Huang X, Xu S, Li L, Wu W, Dai Y, Ge MX, Yuan L, Cao W, Yang M, Wu Y, Deng D (2021) Decreased miR-4512 levels in monocytes and macrophages of individuals with systemic lupus erythematosus contribute to innate immune activation and neutrsophil NETosis by targeting TLR4 and CXCL2. Front Immunol 12:756825. https://doi.org/10.3389/fimmu.2021.756825
Salvador F (2020) ANCA associated vasculitis. Eur J Intern Med 74:18–28. https://doi.org/10.1016/j.ejim.2020.01.011
Hunter RW, Welsh N, Farrah TE, Gallacher PJ, Dhaun N (2020) ANCA associated vasculitis. Bmj 369:m1070. https://doi.org/10.1136/bmj.m1070
Fousert E, Toes R, Desai J (2020) Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells. https://doi.org/10.3390/cells9040915
Masuda S, Nonokawa M, Futamata E, Nishibata Y, Iwasaki S, Tsuji T, Hatanaka Y, Nakazawa D, Tanaka S, Tomaru U, Kawakami T, Atsumi T, Ishizu A (2019) Formation and disordered degradation of neutrophil extracellular traps in necrotizing lesions of anti-neutrophil cytoplasmic antibody-associated vasculitis. Am J Pathol 189:839–846. https://doi.org/10.1016/j.ajpath.2019.01.007
Wang H, Wang C, Zhao MH, Chen M (2015) Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol 181:518–527. https://doi.org/10.1111/cei.12654
Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15:623–625. https://doi.org/10.1038/nm.1959
Panda R, Krieger T, Hopf L, Renné T, Haag F, Röber N, Conrad K, Csernok E, Fuchs TA (2017) Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front Immunol 8:439. https://doi.org/10.3389/fimmu.2017.00439
Kraaij T, Kamerling SWA, van Dam LS, Bakker JA, Bajema IM, Page T, Brunini F, Pusey CD, Toes REM, Scherer HU, Rabelink TJ, van Kooten C, Teng YKO (2018) Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int 94:139–149. https://doi.org/10.1016/j.kint.2018.01.013
Kimura H, Mii A, Shoji J, Arakawa Y, Shimizu A (2021) Immunohistochemical detection of citrullinated histone H3-positive neutrophils is useful for identifying active glomerular and interstitial lesions in antineutrophil cytoplasmic antibody-associated vasculitis. Histopathology 78:520–531. https://doi.org/10.1111/his.14247
Frangou E, Vassilopoulos D, Boletis J, Boumpas DT (2019) An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment. Autoimmun Rev 18:751–760. https://doi.org/10.1016/j.autrev.2019.06.011
Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, Nishio S, Kasahara M, Ishizu A (2012) Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 64:3779–3787. https://doi.org/10.1002/art.34619
Huang YM, Wang H, Wang C, Chen M, Zhao MH (2015) Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol 67:2780–2790. https://doi.org/10.1002/art.39239
Su B, Mao X, Yin B, Chen C, Zhang M, Cui T, Hao Y (2021) TIM-3 regulates the NETs-mediated dendritic cell activation in myeloperoxidase-ANCA-associated vasculitis. Clin Exp Rheumatol 39(2):13–20. https://doi.org/10.55563/clinexprheumatol/6y0bjb
Matsumoto K, Yasuoka H, Yoshimoto K, Suzuki K, Takeuchi T (2021) Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis. Sci Rep 11:222. https://doi.org/10.1038/s41598-020-80685-4
Söderberg D, Segelmark M (2016) Neutrophil extracellular traps in ANCA-associated vasculitis. Front Immunol 7:256. https://doi.org/10.3389/fimmu.2016.00256
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ (2013) NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5:178140. https://doi.org/10.1126/scitranslmed.3005580
Wright HL, Lyon M, Chapman EA, Moots RJ, Edwards SW (2020) Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species, and neutrophil extracellular traps. Front Immunol 11:584116. https://doi.org/10.3389/fimmu.2020.584116
Clarke J (2020) NETs directly injure cartilage in RA. Nat Rev Rheumatol 16:410. https://doi.org/10.1038/s41584-020-0459-4
Chapman EA, Lyon M, Simpson D, Mason D, Beynon RJ, Moots RJ, Wright HL (2019) Caught in a Trap? proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front Immunol 10:423. https://doi.org/10.3389/fimmu.2019.00423
Yamamoto T (2020) Psoriasis and connective tissue diseases. Int J Mol Sci. https://doi.org/10.3390/ijms21165803
Shao S, Fang H, Dang E, Xue K, Zhang J, Li B, Qiao H, Cao T, Zhuang Y, Shen S, Zhang T, Qiao P, Li C, Gudjonsson JE, Wang G (2019) Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front Immunol 10:746. https://doi.org/10.3389/fimmu.2019.00746
Herster F, Bittner Z, Archer NK, Dickhöfer S, Eisel D, Eigenbrod T, Knorpp T, Schneiderhan-Marra N, Löffler MW, Kalbacher H, Vierbuchen T, Heine H, Miller LS, Hartl D, Freund L, Schäkel K, Heister M, Ghoreschi K, Weber ANR (2020) Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat Commun 11:105. https://doi.org/10.1038/s41467-019-13756-4
Kinoshita M, Ogawa Y, Hama N, Ujiie I, Hasegawa A, Nakajima S, Nomura T, Adachi J, Sato T, Koizumi S, Shimada S, Fujita Y, Takahashi H, Mizukawa Y, Tomonaga T, Nagao K, Abe R, Kawamura T (2021) Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aax2398
Li T, Wang C, Liu Y, Li B, Zhang W, Wang L, Yu M, Zhao X, Du J, Zhang J, Dong Z, Jiang T, Xie R, Ma R, Fang S, Zhou J, Shi J (2020) Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns Colitis 14:240–253. https://doi.org/10.1093/ecco-jcc/jjz132
Lai HJ, Doan HT, Lin EY, Chiu YL, Cheng YK, Lin YH, Chiang HS (2023) Histones of neutrophil extracellular traps directly disrupt the permeability and integrity of the intestinal epithelial barrier. Inflamm Bowel Dis 29:783–797. https://doi.org/10.1093/ibd/izac256
Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, Figliuzzi MM, Caprioli F, Stolfi C, Monteleone I, Monteleone G (2019) Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis 13:772–784. https://doi.org/10.1093/ecco-jcc/jjy215
Wang P, Liu D, Zhou Z, Liu F, Shen Y, You Q, Lu S, Wu J (2023) The role of protein arginine deiminase 4-dependent neutrophil extracellular traps formation in ulcerative colitis. Front Immunol 14:1144976. https://doi.org/10.3389/fimmu.2023.1144976
Leppkes M, Lindemann A, Gößwein S, Paulus S, Roth D, Hartung A, Liebing E, Zundler S, Gonzalez-Acera M, Patankar JV, Mascia F, Scheibe K, Hoffmann M, Uderhardt S, Schauer C, Foersch S, Neufert C, Vieth M, Schett G, Atreya R, Kühl AA, Bleich A, Becker C, Herrmann M, Neurath MF (2022) Neutrophils prevent rectal bleeding in ulcerative colitis by peptidyl-arginine deiminase-4-dependent immunothrombosis. Gut 71:2414–2429. https://doi.org/10.1136/gutjnl-2021-324725
Zhang T, Mei Y, Dong W, Wang J, Huang F, Wu J (2020) Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int Immunopharmacol 84:106583. https://doi.org/10.1016/j.intimp.2020.106583
Cao D, Qian K, Zhao Y, Hong J, Chen H, Wang X, Yang N, Zhang C, Cao J, Jia K, Wu G, Zhu M, Shen J, Zhang Y, Cui Z, Wang Z (2023) Association of neutrophil extracellular traps with fistula healing in patients with complex perianal fistulizing crohn’s disease. J Crohns Colitis 17:580–592. https://doi.org/10.1093/ecco-jcc/jjac171
Pierson ER, Wagner CA, Goverman JM (2018) The contribution of neutrophils to CNS autoimmunity. Clin Immunol 189:23–28. https://doi.org/10.1016/j.clim.2016.06.017
Woodberry T, Bouffler SE, Wilson AS, Buckland RL, Brüstle A (2018) The emerging role of neutrophil granulocytes in multiple sclerosis. J Clin Med. https://doi.org/10.3390/jcm7120511
Tillack K, Naegele M, Haueis C, Schippling S, Wandinger KP, Martin R, Sospedra M (2013) Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J Neuroimmunol 261:108–119. https://doi.org/10.1016/j.jneuroim.2013.05.004
Didier K, Giusti D, Le Jan S, Terryn C, Muller C, Pham BN, Le Naour R, Antonicelli FD, Servettaz A (2020) Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis. J Clin Med. https://doi.org/10.3390/jcm9072136
Manfredi AA, Ramirez GA, Godino C, Capobianco A, Monno A, Franchini S, Tombetti E, Corradetti S, Distler JHW, Bianchi ME, Rovere-Querini P, Maugeri N (2022) Platelet phagocytosis via P-selectin glycoprotein ligand 1 and accumulation of microparticles in systemic sclerosis. Arthritis Rheumatol 74:318–328. https://doi.org/10.1002/art.41926
Knight JS, Kanthi Y (2022) Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin Immunopathol. https://doi.org/10.1007/s00281-022-00916-w
Zuo Y, Yalavarthi S, Gockman K, Madison JA, Gudjonsson JE, Kahlenberg JM, Joseph McCune W, Bockenstedt PL, Karp DR, Knight JS (2020) Anti-neutrophil extracellular trap antibodies and impaired neutrophil extracellular trap degradation in antiphospholipid syndrome. Arthritis Rheumatol 72:2130–2135. https://doi.org/10.1002/art.41460
Lu Y, Dong Y, Zhang Y, Shen D, Wang X, Ge R, Zhang M, Xia Y, Wang X (2020) Antiphospholipid antibody-activated NETs exacerbate trophoblast and endothelial cell injury in obstetric antiphospholipid syndrome. J Cell Mol Med 24:6690–6703. https://doi.org/10.1111/jcmm.15321
Arneth B (2021) Defects in ubiquitination and NETosis and their associations with human diseases. Pathology 53:439–445. https://doi.org/10.1016/j.pathol.2020.10.014
Wang X, Yousefi S, Simon HU (2018) Necroptosis and neutrophil-associated disorders. Cell Death Dis 9:111. https://doi.org/10.1038/s41419-017-0058-8
Wang X, Gessier F, Perozzo R, Stojkov D, Hosseini A, Amirshahrokhi K, Kuchen S, Yousefi S, Lötscher P, Simon HU (2020) RIPK3-MLKL-Mediated Neutrophil Death Requires Concurrent Activation of Fibroblast Activation Protein-α. Journal of immunology 205:1653–1663. https://doi.org/10.4049/jimmunol.2000113
Honda T, Yamamoto O, Sawada Y, Egawa G, Kitoh A, Otsuka A, Dainichi T, Nakajima S, Miyachi Y, Kabashima K (2017) Receptor-interacting protein kinase 3 controls keratinocyte activation in a necroptosis-independent manner and promotes psoriatic dermatitis in mice. J Allergy Clin Immunol 140:619-622.e616. https://doi.org/10.1016/j.jaci.2017.02.027
Kril I, Havrylyuk A, Potomkina H, Chopyak V (2020) Apoptosis and secondary necrosis of neutrophils and monocytes in the immunopathogenesis of rheumatoid arthritis: a cohort study. Rheumatol Int 40:1449–1454. https://doi.org/10.1007/s00296-020-04642-0
Zhao J, Jiang P, Guo S, Schrodi SJ, He D (2021) Apoptosis, Autophagy, NETosis, necroptosis, and pyroptosis mediated programmed cell death as targets for innovative therapy in rheumatoid arthritis. Front Immunology 12:809806. https://doi.org/10.3389/fimmu.2021.809806
Wang X, He Z, Liu H, Yousefi S, Simon HU (2016) Neutrophil necroptosis is triggered by ligation of adhesion molecules following GM-CSF priming. J Immunol 197:4090–4100. https://doi.org/10.4049/jimmunol.1600051
Mihalache CC, Yousefi S, Conus S, Villiger PM, Schneider EM, Simon HU (2011) Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J Immunol 186:6532–6542. https://doi.org/10.4049/jimmunol.1004055
Mousavi MJ, Farhadi E, Vodjgani M, Karami J, Tahmasebi MN, Sharafat Vaziri A, Asgari M, Rezaei N, Mostafaei S, Jamshidi A, Mahmoudi M (2021) Role of Fibroblast activation protein alpha in fibroblast-like synoviocytes of rheumatoid arthritis. Iran J Allergy Asthma Immunol 20:338–349. https://doi.org/10.18502/ijaai.v20i3.6335
Schreiber A, Rousselle A, Becker JU, von Mässenhausen A, Linkermann A, Kettritz R (2017) Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci USA 114:E9618-e9625. https://doi.org/10.1073/pnas.1708247114
Weisel K, Berger S, Thorn K, Taylor PC, Peterfy C, Siddall H, Tompson D, Wang S, Quattrocchi E, Burriss SW, Walter J, Tak PP (2021) A randomized, placebo-controlled experimental medicine study of RIPK1 inhibitor GSK2982772 in patients with moderate to severe rheumatoid arthritis. Arthritis Res Ther 23:85. https://doi.org/10.1186/s13075-021-02468-0
Tompson DJ, Davies C, Scott NE, Cannons EP, Kostapanos M, Gross AS, Powell M, Ino H, Shimamura R, Ogura H, Nagakubo T, Igarashi H, Nakano A (2021) Comparison of the Pharmacokinetics of RIPK1 Inhibitor GSK2982772 in Healthy Western and Japanese Subjects. Eur J Drug Metab Pharmacokinet 46:71–83. https://doi.org/10.1007/s13318-020-00652-2
Desai J, Mulay SR, Nakazawa D, Anders HJ (2016) Matters of life and death. How neutrophils die or survive along NET release and is “NETosis” = necroptosis? Cell Mol Life Sci 73:2211–2219. https://doi.org/10.1007/s00018-016-2195-0
Desai J, Kumar SV, Mulay SR, Konrad L, Romoli S, Schauer C, Herrmann M, Bilyy R, Müller S, Popper B, Nakazawa D, Weidenbusch M, Thomasova D, Krautwald S, Linkermann A, Anders HJ (2016) PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol 46:223–229. https://doi.org/10.1002/eji.201545605
Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, Fei K, Jiang G, Fan J (2018) Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis 9:597. https://doi.org/10.1038/s41419-018-0538-5
Pérez-Figueroa E, Álvarez-Carrasco P, Ortega E, Maldonado-Bernal C (2021) Neutrophils: many ways to die. Front Immunol 12:631821. https://doi.org/10.3389/fimmu.2021.631821
Li Z, Guo J, Bi L (2020) Role of the NLRP3 inflammasome in autoimmune diseases. Biomed PharmacotheR 130:110542. https://doi.org/10.1016/j.biopha.2020.110542
Ryu JC, Kim MJ, Kwon Y, Oh JH, Yoon SS, Shin SJ, Yoon JH, Ryu JH (2017) Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol 10:757–774. https://doi.org/10.1038/mi.2016.73
Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, Rathkey JK, Abbott DW, Wang K, Shao F, Catz SD, Dubyak GR, Pearlman E (2020) N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun 11:2212. https://doi.org/10.1038/s41467-020-16043-9
Yi YS (2018) Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J Physiol Pharmacol 22:1–15. https://doi.org/10.4196/kjpp.2018.22.1.1
Kattah NH, Kattah MG, Utz PJ (2010) The U1-snRNP complex: structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol Rev 233:126–145. https://doi.org/10.1111/j.0105-2896.2009.00863.x
Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, Kang I (2012) U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. J Immunol 188:4769–4775. https://doi.org/10.4049/jimmunol.1103355
Huang W, Jiao J, Liu J, Huang M, Hu Y, Ran W, Yan L, Xiong Y, Li M, Quan Z, Rao Y, Chen J, Huang Y, Zhang D (2020) MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov 6:84. https://doi.org/10.1038/s41420-020-00318-7
Tan AL, Marzo-Ortega H, O’Connor P, Fraser A, Emery P, McGonagle D (2004) Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann Rheum Dis 63:1041–1045. https://doi.org/10.1136/ard.2004.020800
Sasaki Y, Otsuka K, Arimochi H, Tsukumo SI, Yasutomo K (2020) Distinct Roles of IL-1β and IL-18 in NLRC4-Induced Autoinflammation. Front Immunol 11:591713. https://doi.org/10.3389/fimmu.2020.591713
Hu Q, Shi H, Zeng T, Liu H, Su Y, Cheng X, Ye J, Yin Y, Liu M, Zheng H, Wu X, Chi H, Zhou Z, Jia J, Sun Y, Teng J, Yang C (2019) Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still’s disease. Arthritis Res Ther 21:9. https://doi.org/10.1186/s13075-018-1800-z
Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K (2018) Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol. https://doi.org/10.1126/sciimmunol.aar6676
Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Krüger R, Herzig A, Zychlinsky A (2018) Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. https://doi.org/10.1126/sciimmunol.aar6689
Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, Silberstein LE, Cheng T, Han M, Xu Y, Luo HR (2018) Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep 22:2924–2936. https://doi.org/10.1016/j.celrep.2018.02.067
Yang Z, Cao J, Yu C, Yang Q, Zhang Y, Han L (2016) Caspase-1 mediated interleukin-18 activation in neutrophils promotes the activity of rheumatoid arthritis in a NLRP3 inflammasome independent manner. Joint Bone Spine 83:282–289. https://doi.org/10.1016/j.jbspin.2015.07.006
Xie Z, Hou H, Luo D, An R, Zhao Y, Qiu C (2021) ROS-Dependent Lipid peroxidation and reliant antioxidant ferroptosis-suppressor-protein 1 in rheumatoid arthritis: a covert clue for potential therapy. Inflammation 44:35–47. https://doi.org/10.1007/s10753-020-01338-2
Qiu Y, Cao Y, Cao W, Jia Y, Lu N (2020) The Application of Ferroptosis in Diseases. Pharmacol Res 159:104919. https://doi.org/10.1016/j.phrs.2020.104919
Jakaria M, Belaidi AA, Bush AI, Ayton S (2021) Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J Neurochem 159:804–825. https://doi.org/10.1111/jnc.15519
Zhang G, Zhang Y, Shen Y, Wang Y, Zhao M, Sun L (2021) The potential role of ferroptosis in alzheimer’s disease. J Alzheimers Dis 80:907–925. https://doi.org/10.3233/jad-201369
Xiong R, He R, Liu B, Jiang W, Wang B, Li N, Geng Q (2021) Ferroptosis: a new promising target for lung cancer therapy. Oxid Med Cell Longev 2021:8457521. https://doi.org/10.1155/2021/8457521
Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F (2020) Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. https://doi.org/10.3390/cells9061505
Chen X, Kang R, Kroemer G, Tang D (2021) Ferroptosis in infection, inflammation, and immunity. J Exp Med. https://doi.org/10.1084/jem.20210518
Seibt TM, Proneth B, Conrad M (2019) Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 133:144–152. https://doi.org/10.1016/j.freeradbiomed.2018.09.014
Deng G, Li Y, Ma S, Gao Z, Zeng T, Chen L, Ye H, Yang M, Shi H, Yao X, Zeng Z, Chen Y, Song Y, Liu B, Gao L (2020) Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic Biol Med 148:151–161. https://doi.org/10.1016/j.freeradbiomed.2019.12.026
Li P, Jiang M, Li K, Li H, Zhou Y, Xiao X, Xu Y, Krishfield S, Lipsky PE, Tsokos GC, Zhang X (2021) Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol 22:1107–1117. https://doi.org/10.1038/s41590-021-00993-3
Ohl K, Rauen T, Tenbrock K (2021) Dysregulated neutrophilic cell death in SLE: a spotlight on ferroptosis. Signal Transduct Target Ther 6:392. https://doi.org/10.1038/s41392-021-00804-z
Skendros P, Mitroulis I, Ritis K (2018) Autophagy in neutrophils: from granulopoiesis to neutrophil extracellular traps. Front Cell Dev Biol 6:109. https://doi.org/10.3389/fcell.2018.00109
Sidaway P (2017) Neutrophils: neutrophil differentiation is autophagy dependent. Nat Rev Immunol 17:662. https://doi.org/10.1038/nri.2017.122
Riffelmacher T, Clarke A, Richter FC, Stranks A, Pandey S, Danielli S, Hublitz P, Yu Z, Johnson E, Schwerd T, McCullagh J, Uhlig H, Jacobsen SEW, Simon AK (2017) Autophagy-dependent generation of free fatty acids is critical for normal neutrophil differentiation. Immunity 47:466-480.e465. https://doi.org/10.1016/j.immuni.2017.08.005
Bhattacharya A, Wei Q, Shin JN, Abdel Fattah E, Bonilla DL, Xiang Q, Eissa NT (2015) Autophagy is required for neutrophil-mediated inflammation. Cell Rep 12:1731–1739. https://doi.org/10.1016/j.celrep.2015.08.019
Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, Maiuri L, Maseri A, D’Angelo A, Bianchi ME, Rovere-Querini P, Manfredi AA (2014) Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost 12:2074–2088. https://doi.org/10.1111/jth.12710
Manfredi AA, Rovere-Querini P, D’Angelo A, Maugeri N (2017) Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol Res 123:146–156. https://doi.org/10.1016/j.phrs.2016.08.008
Murthy P, Singhi AD, Ross MA, Loughran P, Paragomi P, Papachristou GI, Whitcomb DC, Zureikat AH, Lotze MT, Zeh Iii HJ, Boone BA (2019) Enhanced neutrophil extracellular trap formation in acute pancreatitis contributes to disease severity and is reduced by chloroquine. Front Immunol 10:28. https://doi.org/10.3389/fimmu.2019.00028
Ma R, Li T, Cao M, Si Y, Wu X, Zhao L, Yao Z, Zhang Y, Fang S, Deng R, Novakovic VA, Bi Y, Kou J, Yu B, Yang S, Wang J, Zhou J, Shi J (2016) Extracellular DNA traps released by acute promyelocytic leukemia cells through autophagy. Cell Death Dis 7:e2283. https://doi.org/10.1038/cddis.2016.186
Mulcahy ME, O’Brien EC, O’Keeffe KM, Vozza EG, Leddy N, McLoughlin RM (2020) Manipulation of Autophagy and Apoptosis Facilitates Intracellular Survival of Staphylococcus aureus in Human Neutrophils. Front Immunol 11:565545. https://doi.org/10.3389/fimmu.2020.565545
Pliyev BK, Menshikov M (2012) Differential effects of the autophagy inhibitors 3-methyladenine and chloroquine on spontaneous and TNF-α-induced neutrophil apoptosis. Apoptosis 17:1050–1065. https://doi.org/10.1007/s10495-012-0738-x
Iula L, Keitelman IA, Sabbione F, Fuentes F, Guzman M, Galletti JG, Gerber PP, Ostrowski M, Geffner JR, Jancic CC, Trevani AS (2018) Autophagy mediates interleukin-1β secretion in human neutrophils. Front Immunol 9:269. https://doi.org/10.3389/fimmu.2018.00269
Frangou E, Chrysanthopoulou A, Mitsios A, Kambas K, Arelaki S, Angelidou I, Arampatzioglou A, Gakiopoulou H, Bertsias GK, Verginis P, Ritis K, Boumpas DT (2019) REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis 78:238–248. https://doi.org/10.1136/annrheumdis-2018-213181
Angelidou I, Chrysanthopoulou A, Mitsios A, Arelaki S, Arampatzioglou A, Kambas K, Ritis D, Tsironidou V, Moschos I, Dalla V, Stakos D, Kouklakis G, Mitroulis I, Ritis K, Skendros P (2018) REDD1/autophagy pathway is associated with neutrophil-driven IL-1β inflammatory response in active ulcerative colitis. J Immunol 200:3950–3961. https://doi.org/10.4049/jimmunol.1701643
Tang S, Zhang Y, Yin SW, Gao XJ, Shi WW, Wang Y, Huang X, Wang L, Zou LY, Zhao JH, Huang YJ, Shan LY, Gounni AS, Wu YZ, Zhang JB (2015) Neutrophil extracellular trap formation is associated with autophagy-related signalling in ANCA-associated vasculitis. Clin Exp Immunol 180:408–418. https://doi.org/10.1111/cei.12589
Maugeri N, Capobianco A, Rovere-Querini P, Ramirez GA, Tombetti E, Valle PD, Monno A, D’Alberti V, Gasparri AM, Franchini S, D’Angelo A, Bianchi ME, Manfredi AA (2018) Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao3089
Suzuki E, Maverakis E, Sarin R, Bouchareychas L, Kuchroo VK, Nestle FO, Adamopoulos IE (2016) T Cell-independent mechanisms associated with neutrophil extracellular trap formation and selective autophagy in IL-17A-Mediated epidermal hyperplasia. J Immunol 197:4403–4412. https://doi.org/10.4049/jimmunol.1600383
Speir M, Nowell CJ, Chen AA, O’Donnell JA, Shamie IS, Lakin PR, D’Cruz AA, Braun RO, Babon JJ, Lewis RS, Bliss-Moreau M, Shlomovitz I, Wang S, Cengia LH, Stoica AI, Hakem R, Kelliher MA, O’Reilly LA, Patsiouras H, Lawlor KE, Weller E, Lewis NE, Roberts AW, Gerlic M, Croker BA (2020) Ptpn6 inhibits caspase-8- and Ripk3/Mlkl-dependent inflammation. Nat Immunol 21:54–64. https://doi.org/10.1038/s41590-019-0550-7
Soni C, Reizis B (2019) Self-DNA at the Epicenter of SLE: Immunogenic Forms, Regulation, and Effects. Front Immunol 10:1601. https://doi.org/10.3389/fimmu.2019.01601
Mahajan A, Herrmann M, Muñoz LE (2016) Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front Immunol 7:35. https://doi.org/10.3389/fimmu.2016.00035
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE (2016) New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 12:716–730. https://doi.org/10.1038/nrrheum.2016.186
Huang W, Wu J, Yang H, Xiong Y, Jiang R, Cui T, Ye D (2017) Milk fat globule-EGF factor 8 suppresses the aberrant immune response of systemic lupus erythematosus-derived neutrophils and associated tissue damage. Cell Death Differ 24:263–275. https://doi.org/10.1038/cdd.2016.115
Lauber K, Keppeler H, Munoz LE, Koppe U, Schröder K, Yamaguchi H, Krönke G, Uderhardt S, Wesselborg S, Belka C, Nagata S, Herrmann M (2013) Milk fat globule-EGF factor 8 mediates the enhancement of apoptotic cell clearance by glucocorticoids. Cell Death Differ 20:1230–1240. https://doi.org/10.1038/cdd.2013.82
Stefanescu M, Matache C, Onu A, Tanaseanu S, Dragomir C, Constantinescu I, Schönlau F, Rohdewald P, Szegli G (2001) Pycnogenol efficacy in the treatment of systemic lupus erythematosus patients. Phytother Res 15:698–704. https://doi.org/10.1002/ptr.915
Wehrli M, Schneider C, Cortinas-Elizondo F, Verschoor D, Frias Boligan K, Adams OJ, Hlushchuk R, Engelmann C, Daudel F, Villiger PM, Seibold F, Yawalkar N, Vonarburg C, Miescher S, Lötscher M, Kaufmann T, Münz C, Mueller C, Djonov V, Simon HU, von Gunten S (2020) IgA triggers cell death of neutrophils when primed by inflammatory mediators. J Immunol 205:2640–2648. https://doi.org/10.4049/jimmunol.1900883
Graeter S, Schneider C, Verschoor D, von Däniken S, Seibold F, Yawalkar N, Villiger P, Dimitrov JD, Smith DF, Cummings RD, Simon HU, Vassilev T, von Gunten S (2020) Enhanced pro-apoptotic effects of Fe(II)-modified IVIG on human neutrophils. Front Immunol 11:973. https://doi.org/10.3389/fimmu.2020.00973
Watanabe-Kusunoki K, Nakazawa D, Kusunoki Y, Kudo T, Hattanda F, Nishio S, Masuda S, Tomaru U, Kondo T, Atsumi T, Ishizu A (2020) Recombinant thrombomodulin ameliorates autoimmune vasculitis via immune response regulation and tissue injury protection. J Autoimmun 108:102390. https://doi.org/10.1016/j.jaut.2019.102390
Uozumi R, Iguchi R, Masuda S, Nishibata Y, Nakazawa D, Tomaru U, Ishizu A (2020) Pharmaceutical immunoglobulins reduce neutrophil extracellular trap formation and ameliorate the development of MPO-ANCA-associated vasculitis. Modern Rheumatol 30:544–550. https://doi.org/10.1080/14397595.2019.1602292
Fortner KA, Blanco LP, Buskiewicz I, Huang N, Gibson PC, Cook DL, Pedersen HL, Yuen PST, Murphy MP, Perl A, Kaplan MJ, Budd RC (2020) Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med. https://doi.org/10.1136/lupus-2020-000387
Hair PS, Enos AI, Krishna NK, Cunnion KM (2018) Inhibition of immune complex complement activation and neutrophil extracellular trap formation by peptide inhibitor of complement C1. Front Immunol 9:558. https://doi.org/10.3389/fimmu.2018.00558
Yu Y, Koehn CD, Yue Y, Li S, Thiele GM, Hearth-Holmes MP, Mikuls TR, O’Dell JR, Klassen LW, Zhang Z, Su K (2015) Celastrol inhibits inflammatory stimuli-induced neutrophil extracellular trap formation. Curr Mol Med 15:401–410. https://doi.org/10.2174/1566524015666150505160743
Liao P, He Y, Yang F, Luo G, Zhuang J, Zhai Z, Zhuang L, Lin Z, Zheng J, Sun E (2018) Polydatin effectively attenuates disease activity in lupus-prone mouse models by blocking ROS-mediated NET formation. Arthritis Res Ther 20:254. https://doi.org/10.1186/s13075-018-1749-y
Yang F, Luo X, Luo G, Zhai Z, Zhuang J, He J, Han J, Zhang Y, Zhuang L, Sun E, He Y (2019) Inhibition of NET formation by polydatin protects against collagen-induced arthritis. Int Immunopharmacol 77:105919. https://doi.org/10.1016/j.intimp.2019.105919
Furumoto Y, Smith CK, Blanco L, Zhao W, Brooks SR, Thacker SG, Abdalrahman Z, Sciumè G, Tsai WL, Trier AM, Nunez L, Mast L, Hoffmann V, Remaley AT, O’Shea JJ, Kaplan MJ, Gadina M (2017) Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol 69:148–160. https://doi.org/10.1002/art.39818
Kraaij T, Kamerling SWA, de Rooij ENM, van Daele PLA, Bredewold OW, Bakker JA, Bajema IM, Scherer HU, Toes REM, Huizinga TJW, Rabelink TJ, van Kooten C, Teng YKO (2018) The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun 91:45–54. https://doi.org/10.1016/j.jaut.2018.03.003
Pérez-Sánchez C, Ruiz-Limón P, Aguirre MA, Jiménez-Gómez Y, Arias-de la Rosa I, Ábalos-Aguilera MC, Rodriguez-Ariza A, Castro-Villegas MC, Ortega-Castro R, Segui P, Martinez C, Gonzalez-Conejero R, Rodríguez-López S, Gonzalez-Reyes JA, Villalba JM, Collantes-Estévez E, Escudero A, Barbarroja N, López-Pedrera C (2017) Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. J Autoimmun 82:31–40. https://doi.org/10.1016/j.jaut.2017.04.007
Yuan K, Zhu Q, Lu Q, Jiang H, Zhu M, Li X, Huang G, Xu A (2020) Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J Nutr Biochem 84:108454. https://doi.org/10.1016/j.jnutbio.2020.108454
Zhang S, Huang G, Yuan K, Zhu Q, Sheng H, Yu R, Luo G, Xu A (2017) Tanshinone IIA ameliorates chronic arthritis in mice by modulating neutrophil activities. Clin Exp Immunol 190:29–39. https://doi.org/10.1111/cei.12993
Fukui S, Gutch S, Fukui S, Chu L, Wagner DD (2022) Anti-inflammatory protective effect of ADAMTS-13 in murine arthritis models. J Thromb Haemost 20:2386–2393. https://doi.org/10.1111/jth.15828
Funding
This work was funded by the National Natural Science Foundation of China (No.82204403); China Postdoctoral Science Foundation (No.2022M72199); Key Research and Development Plan of Anhui Province in 2021-Population Health Project (202104j07020032) and open project of Key Laboratory of Anti-inflammatory Immune Drugs of Ministry of Education (KFJJ-2021-07).
Author information
Authors and Affiliations
Contributions
ZW ZHANG and L JIN: preparing the original manuscript; LH LIU and MQ ZHOU: preparing the figures; XZ ZHANG and LL ZHANG: performing manuscript review and revision. All authors have read and approved the final draft of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors approve the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Jin, L., Liu, L. et al. The intricate relationship between autoimmunity disease and neutrophils death patterns: a love-hate story. Apoptosis 28, 1259–1284 (2023). https://doi.org/10.1007/s10495-023-01874-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01874-w