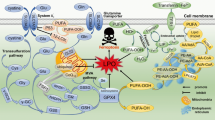
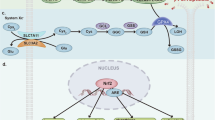
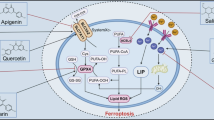
Abstract
It has been 10 years since the concept of ferroptosis was put forward and research focusing on ferroptosis has been increasing continuously. Ferroptosis is driven by iron-dependent lipid peroxidation, which can be antagonized by glutathione peroxidase 4 (GPX4), ferroptosis inhibitory protein 1 (FSP1), dihydroorotate dehydrogenase (DHODH) and Fas-associated factor 1 (FAF1). Various cellular metabolic events, including lipid metabolism, can modulate ferroptosis sensitivity. It is worth noting that the reprogramming of lipid metabolism in cancer cells can promote the occurrence and development of tumors. The metabolic flexibility of cancer cells opens the possibility for the coordinated targeting of multiple lipid metabolic pathways to trigger cancer cells ferroptosis. In addition, cancer cells must obtain immortality, escape from programmed cell death including ferroptosis, to promote cancer progression, which provides new perspectives for improving cancer therapy. Targeting the vulnerability of ferroptosis has received attention as one of the significant possible strategies to treat cancer given its role in regulating tumor cell survival. We review the impact of iron and lipid metabolism on ferroptosis and the potential role of the crosstalk of lipid metabolism reprogramming and ferroptosis in antitumor immunity and sum up agents targeting lipid metabolism and ferroptosis for cancer therapy.




Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- GPX4:
-
Glutathione peroxidase 4
- FSP1:
-
Ferroptosis inhibitory protein 1
- DHODH:
-
Dihydroorotate dehydrogenase
- FAF1:
-
Fas-associated factor 1
- PUFAs:
-
Polyunsaturated fatty acids
- ROS:
-
Reactive oxygen species
- LIP:
-
Labile iron pool
- LOXs:
-
Lipoxygenases
- POR:
-
Cytochrome P450 oxidoreductase
- CSCs:
-
Cancer stem cells
- TF:
-
Transferrin
- TFRC:
-
Transferrin receptor
- CTCs:
-
Circulating tumor cells
- DMT1:
-
Divalent metal transporter 1
- NCOA4:
-
Nuclear receptor coactivator 4
- GOT1:
-
Cytosolic aspartate aminotransaminase
- HMOX1:
-
Heme oxygenase 1
- NFS1:
-
Nitrogenfixation 1
- PCBP2:
-
Poly rC binding-protein 2
- AA:
-
Arachidonoyl
- AdA:
-
Adrenoyl
- ACSL4:
-
Acyl-CoA synthetase long-chain family member 4
- PEs:
-
Phosphatidylethanolamines
- MUFAs:
-
Monounsaturated fatty acids
- POA:
-
Palmitic acid
- OA:
-
Oleic acid
- ACSL3:
-
Acyl-CoA synthetase long chain family member 3
- SFA:
-
Saturated fatty acyl
- PUFA ePLs:
-
Polyunsaturated ether phospholipids
- LPCAT3:
-
Lysophosphatidylcholine acyltransferase 3
- PKCβII:
-
Protein kinase C-βII isoform
- PEBP1:
-
Phosphatidylethanolamine-binding protein 1
- 4-HNE:
-
4-Hydroxynonenal
- MDA:
-
Malondialdehyde
- CoQ10:
-
Coenzyme Q10
- a-TOC:
-
Alpha-tocopherol
- CoQ:
-
Ubiquinone
- CoQH2:
-
Ubiquinol
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- Sec:
-
Selenocysteine
- TFH:
-
Follicular helper T cells
- TFAP2C:
-
Transcription factor AP-2γ
- SP1:
-
Specific protein 1
- GSH:
-
Glutathione
- GCL:
-
Glutamate-cysteineligase
- BSO:
-
Butionine sulfoximine
- GCLC:
-
Glutamic acid cysteine ligase catalytic subunit
- BAP1:
-
BRCA1-associated protein 1
- CSLCs:
-
Cancer stem cell-like cells
- BCSCs:
-
Breast cancer stem cells
- CAFs:
-
Cancer-associated fibroblasts
- MESH1:
-
Metazoan SpoT Homolog 1
- ER:
-
Endoplasmic reticulum
- AIFM2:
-
Apoptosis-inducing factor mitochondria-associated 2
- AIF:
-
Apoptosis inducing factor
- NDH-2:
-
Type 2 NADH ubiquinone oxidoreductase
- IPP:
-
Isopentenyl pyrophosphate
- BH4:
-
Tetrahydrobiopterin
- DHFR:
-
Dihydrofolate reductase
- BH2:
-
Dihydrobiopterin
- GCH1:
-
GTP-dependent cyclohydrolase 1
- iPLA2β:
-
Calcium-independent phospholipase A2β
- 27HC:
-
27-Hydroxycholesterol
- SQS:
-
Squalene synthase
- SQLE:
-
Squalene monooxygenase
- ACSF2:
-
Acyl-CoA synthetase family member 2
- CS:
-
Citrate synthase
- SCD1:
-
Stearoyl-CoA desaturase 1
- FADS2:
-
Acyl-CoA 6 desaturase
- CSCs:
-
Cancer stem cells
- SREBPs:
-
Sterol regulatory element binding proteins
- BCAT2:
-
Branched-chain amino acid aminotransferase 2
- AMPK:
-
AMP-activated protein kinase
- LKB1:
-
Liver kinase B1
- FAT:
-
Fatty acid translocase
- LDs:
-
Lipid droplets
- DGATi:
-
Diacylglycerol acyltransferase inhibitor
- TPD52:
-
Tumor protein D52
- HILPDA:
-
Hypoxia inducible lipid droplet-associated
- DAMPs:
-
Damage associated molecular patterns
- VLDLRs:
-
Very low-density lipoproteins
- LDLRs:
-
LDL receptors
- LSRs:
-
Ipolysis-stimulating receptors
- ACLY:
-
ATP-citrate lyase
- ACSS2:
-
Acyl-CoA synthetase short chain family member 2
- ACC:
-
Acetyl-CoA carboxylase
- USP22:
-
Ubiquitin-specific enzyme 22
- FADS:
-
FA desaturase
- ELOVL:
-
Elongating very long-chain fatty acid enzyme
- GCs:
-
Gastric cancer cells
- HMGCRs:
-
HMG-CoA reductases
- SQLE:
-
Squalene epoxidase
- ALCLs:
-
Anaplastic large cell lymphomas
- EMT:
-
Epithelial-mesenchymal transition
- FAO:
-
Fatty acid oxidation
- TAGs:
-
Triacylglycerols
- CEs:
-
Cholesteryl esters
- DGAT1:
-
Diacylglycerol-acyltransferase 1
- MGL:
-
Monoacylglycerol lipase
- ATGL:
-
Lipase fat triglyceride lipase
- HSL:
-
Hormone-sensitive lipase
- TME:
-
Tumor microenvironment
- STAT3:
-
Transcription 3
- ALOX15:
-
Arachidonic acid lipoxygenase 15
- STAT1:
-
Transcription 1
- PGE2:
-
Prostaglandin E2
- cDC1:
-
Conventional type 1 dendritic cells
- NK:
-
Natural killer
- OXPLs:
-
Oxidized phospholipids
- TLR2:
-
Toll-like receptor 2
- iNOS:
-
Inducible nitric oxide synthase
- MZ:
-
Marginal zone
- ICD:
-
Immunogenic cell death
- HMGB1:
-
High mobility histone B1
- AGER:
-
Advanced glycosylation end-product specific receptor
- PDT:
-
Photodynamic therapy
- BMDCs:
-
Bone marrow-derived dendritic cells
- TAMs:
-
Tumor associated macrophages
- HCC:
-
Hepatocellular carcinoma
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- NSCLC:
-
Non-small cell lung cancer
- BetA:
-
Betulinic acid
- SCLC:
-
Small-cell lung cancer
- HDLNPs:
-
HDL-like nanoparticle
- SCARB1:
-
Scavenger receptor class B member 1
- INSIG1:
-
Insulin induced gene 1
- HMGCS1:
-
HMG-CoA synthase 1
- IKE:
-
Imidazole-ketone-erastin
References
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285. https://doi.org/10.1016/j.cell.2017.09.021
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al (2014) Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 156(1–2):317–331. https://doi.org/10.1016/j.cell.2013.12.010
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold L et al (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575(7784):693. https://doi.org/10.1038/s41586-019-1707-0
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692. https://doi.org/10.1038/s41586-019-1705-2
Mao C, Liu XG, Zhang YL, Lei G, Yan YL, Lee H al (2021) DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593(7860):586–. https://doi.org/10.1038/s41586-021-03539-7
Cui S, Simmons G Jr, Vale G, Deng Y, Kim J, Kim H al (2022) FAF1 blocks ferroptosis by inhibiting peroxidation of polyunsaturated fatty acids. Proc Natl Acad Sci U S A 119(17):e2107189119. https://doi.org/10.1073/pnas.2107189119
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, al, (2020) Ferroptosis: past, present and future. Cell Death Dis. https://doi.org/10.1038/s41419-020-2298-2
Stine ZE, Schug ZT, Salvino JM, Dang CV (2022) Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discovery 21(2):141–162. https://doi.org/10.1038/s41573-021-00339-6
Koundouros N, Poulogiannis G (2020) Reprogramming of fatty acid metabolism in cancer. Br J Cancer 122(1):4–22. https://doi.org/10.1038/s41416-019-0650-z
Zheng J, Conrad M (2020) The metabolic underpinnings of ferroptosis. Cell Metab 32(6):920–937. https://doi.org/10.1016/j.cmet.2020.10.011
Zou Y, Palte MJ, Deik AA, Li HX, Eaton JK, Wang WY et al (2019) A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. https://doi.org/10.1038/s41467-019-09277-9
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26(3):165–176. https://doi.org/10.1016/j.tcb.2015.10.014
Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35(6):830–849. https://doi.org/10.1016/j.ccell.2019.04.002
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A al (2017) Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551(7679):247–250. https://doi.org/10.1038/nature24297
Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y al (2022) CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40(4):365–378e366. https://doi.org/10.1016/j.ccell.2022.02.003
Chen X, Kang R, Kroemer G, Tang D (2021) Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 18(5):280–296. https://doi.org/10.1038/s41571-020-00462-0
Liu Y, Duan C, Dai R, Zeng Y (2021) Ferroptosis-mediated crosstalk in the tumor microenvironment implicated in cancer progression and therapy. Front Cell Dev Biol 9:739392. https://doi.org/10.3389/fcell.2021.739392
Winterbourn CC (1995) Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 82–83:969–974. https://doi.org/10.1016/0378-4274(95)03532-x
Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM (2018) Iron and Cancer. Annu Rev Nutr 38:97–125. https://doi.org/10.1146/annurev-nutr-082117-051732
Doll S, Conrad M (2017) Iron and ferroptosis: a still ill-defined liaison. IUBMB Life 69(6):423–434. https://doi.org/10.1002/iub.1616
Rodriguez R, Schreiber SL, Conrad M (2022) Persister cancer cells: iron addiction and vulnerability to ferroptosis. Mol Cell 82(4):728–740. https://doi.org/10.1016/j.molcel.2021.12.001
Recalcati S, Gammella E, Cairo G (2019) Dysregulation of iron metabolism in cancer stem cells. Free Radic Biol Med 133:216–220. https://doi.org/10.1016/j.freeradbiomed.2018.07.015
Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM et al (2020) Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep 30(10):3411. https://doi.org/10.1016/j.celrep.2020.02.049
Hong X, Roh W, Sullivan RJ, Wong KHK, Wittner BS, Guo H et al (2021) The lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses ferroptosis. Cancer Discov 11(3):678–695. https://doi.org/10.1158/2159-8290.CD-19-1500
El Hout M, Dos Santos L, Hamai A, Mehrpour M (2018) A promising new approach to cancer therapy: targeting iron metabolism in cancer stem cells. Sem Cancer Biol 53:125–138. https://doi.org/10.1016/j.semcancer.2018.07.009
Gao MH, Monian P, Pan QH, Zhang W, Xiang J, Jiang XJ (2016) Ferroptosis is an autophagic cell death process. Cell Res 26(9):1021–1032. https://doi.org/10.1038/cr.2016.95
Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Sasaki M al (2016) An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem J 473:769–777. https://doi.org/10.1042/Bj20150658
Hou W, Xie YC, Song XX, Sun XF, Lotze MT, Zeh HJet al (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12(8):1425–1428. https://doi.org/10.1080/15548627.2016.1187366
Rizzollo F, More S, Vangheluwe P, Agostinis P (2021) The lysosome as a master regulator of iron metabolism. Trends Biochem Sci 46(12):960–975. https://doi.org/10.1016/j.tibs.2021.07.003
Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA et al (2021) GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun. https://doi.org/10.1038/s41467-021-24859-2
Sun XF, Ou ZH, Chen RC, Niu XH, Chen D, Kang R al (2016) Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63(1):173–184. https://doi.org/10.1002/hep.28251
Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC (2018) Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett 416:124–137. https://doi.org/10.1016/j.canlet.2017.12.025
Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S et al (2017) NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551(7682):639–643. https://doi.org/10.1038/nature24637
Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu HB, Zhu LJ et al (2019) Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell 51(5):575. https://doi.org/10.1016/j.devcel.2019.10.007
Ganz T (2005) Cellular iron: ferroportin is the only way out. Cell Metab 1(3):155–157. https://doi.org/10.1016/j.cmet.2005.02.005
Drakesmith H, Nemeth E, Ganz T (2015) Ironing out ferroportin. Cell Metab 22(5):777–787. https://doi.org/10.1016/j.cmet.2015.09.006
Yang QY, Liu W, Zhang SP, Liu SJ (2020) The cardinal roles of ferroportin and its partners in controlling cellular iron in and out. Life Sci. https://doi.org/10.1016/j.lfs.2020.118135
Tang Z, Jiang W, Mao M, Zhao J, Chen J, Cheng N (2021) Deubiquitinase USP35 modulates ferroptosis in lung cancer via targeting ferroportin. Clin Transl Med 11(4):e390. https://doi.org/10.1002/ctm2.390
Li Y, Zeng X, Lu D, Yin M, Shan M, Gao Y (2021) Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum Reprod 36(4):951–964. https://doi.org/10.1093/humrep/deaa363
Yanatori I, Richardson DR, Imada K, Kishi F (2016) Iron export through the transporter ferroportin 1 is modulated by the iron chaperone PCBP2. J Biol Chem 291(33):17303–17318. https://doi.org/10.1074/jbc.M116.721936
De Bose-Boyd RA (2018) Significance and regulation of lipid metabolism. Semin Cell Dev Biol 81:97. https://doi.org/10.1016/j.semcdb.2017.12.003
Beloribi-Djefaflia S, Vasseur S, Guillaumond F (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5:e189
Stockwell BR (2022) Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 185(14):2401–2421. https://doi.org/10.1016/j.cell.2022.06.003
Liang DG, Minikes AM, Jiang XJ (2022) Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell 82(12):2215–2227. https://doi.org/10.1016/j.molcel.2022.03.022
Lee JY, Kim WK, Bae KH, Lee SC, Lee EW (2021) Lipid metabolism and ferroptosis. Biology-Basel. https://doi.org/10.3390/biology10030184
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90. https://doi.org/10.1038/nchembio.2238
Rouzer CA, Marnett LJ (2003) Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev 103(6):2239–2304. https://doi.org/10.1021/cr000068x
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B al (2017) Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547(7664):453–457. https://doi.org/10.1038/nature23007
Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W al (2020) Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16(3):302–309. https://doi.org/10.1038/s41589-020-0472-6
Kathman SG, Boshart J, Jing H, Cravatt BF (2020) Blockade of the lysophosphatidylserine lipase ABHD12 potentiates ferroptosis in cancer cells. ACS Chem Biol 15(4):871–877. https://doi.org/10.1021/acschembio.0c00086
Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P al (2020) Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585(7826):603–608. https://doi.org/10.1038/s41586-020-2732-8
Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS et al (2020) Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585(7823):113–118. https://doi.org/10.1038/s41586-020-2623-z
Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A et al (2019) Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol 26(3):420-432e429. https://doi.org/10.1016/j.chembiol.2018.11.016
Hoy AJ, Nagarajan SR, Butler LM (2021) Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer 21(12):753–766. https://doi.org/10.1038/s41568-021-00388-4
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98. https://doi.org/10.1038/nchembio.2239
Reed A, Ichu TA, Milosevich N, Melillo B, Schafroth MA, Otsuka Y et al (2022) LPCAT3 inhibitors remodel the polyunsaturated phospholipid content of human cells and protect from ferroptosis. ACS Chem Biol 17(6):1607–1618. https://doi.org/10.1021/acschembio.2c00317
Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR et al (2019) Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572(7769):402–406. https://doi.org/10.1038/s41586-019-1426-6
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282. https://doi.org/10.1038/s41580-020-00324-8
Sha R, Xu Y, Yuan C, Sheng X, Wu Z, Peng J et al (2021) Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine 71:103560. https://doi.org/10.1016/j.ebiom.2021.103560
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA et al (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6(1):49. https://doi.org/10.1038/s41392-020-00428-9
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL, Cheng K al (2018) Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res 28(12):1171–1185. https://doi.org/10.1038/s41422-018-0090-y
Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye ZP et al (2022) PKC beta II phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol 24(1):88. https://doi.org/10.1038/s41556-021-00818-3
Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G (2017) PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171(3):628-641e626. https://doi.org/10.1016/j.cell.2017.09.044
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113(34):E4966–4975. https://doi.org/10.1073/pnas.1603244113
Gardner HW (1989) Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med 7(1):65–86. https://doi.org/10.1016/0891-5849(89)90102-0
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438. https://doi.org/10.1155/2014/360438
Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L (2007) Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J 93(12):4225–4236. https://doi.org/10.1529/biophysj.107.112565
Chng CP, Sadovsky Y, Hsia KJ, Huang C (2021) Site-specific peroxidation modulates lipid bilayer mechanics. Extreme Mech Lett. https://doi.org/10.1016/j.eml.2020.101148
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F (2020) GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. Acs Cent Sci 6(1):41–53. https://doi.org/10.1021/acscentsci.9b01063
Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T (2017) Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol 403:143–170. https://doi.org/10.1007/82_2016_508
Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K (2018) Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172(3):409. https://doi.org/10.1016/j.cell.2017.11.048
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radical Bio Med 152:175–185
Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F (2020) Ferroptosis and cancer: mitochondria meet the “Iron Maiden” cell death. Cells. https://doi.org/10.3390/cells9061505
Yao Y, Chen Z, Zhang H, Chen CL, Zeng M, Yunis J al (2021) Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol 22(9):1127–. https://doi.org/10.1038/s41590-021-00996-0
Alim I, Caulfield JT, Chen YX, Swarup V, Geschwind DH, Ivanova E et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262. https://doi.org/10.1016/j.cell.2019.03.032
Li Y, Feng DC, Wang ZY, Zhao Y, Sun RM, Tian DH et al (2019) Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 26(11):2284–2299. https://doi.org/10.1038/s41418-019-0299-4
Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A et al (2016) An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 29(1):104–116. https://doi.org/10.1016/j.ccell.2015.12.004
Nishizawa S, Araki H, Ishikawa Y, Kitazawa S, Hata A, Soga T al (2018) Low tumor glutathione level as a sensitivity marker for glutamate-cysteine ligase inhibitors. Oncol Lett 15(6):8735–8743. https://doi.org/10.3892/ol.2018.8447
Stockwell BR (2018) Ferroptosis: death by lipid peroxidation. Free Radical Bio Med 120:S7–S7. https://doi.org/10.1016/j.freeradbiomed.2018.04.034
Gao MH, Monian P, Quadri N, Ramasamy R, Jiang XJ (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308. https://doi.org/10.1016/j.molcel.2015.06.011
Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V al (2020) Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368(6486):85–. https://doi.org/10.1126/science.aaw9872
Alborzinia H, Florez AF, Kreth S, Bruckner LM, Yildiz U, Gartlgruber M al (2022) MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat Cancer 3(4):471–485. https://doi.org/10.1038/s43018-022-00355-4
Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E (2021) Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab 33(1):174-189e177. https://doi.org/10.1016/j.cmet.2020.12.007
Zhang YL, Shi JJ, Liu XG, Feng L, Gong ZH, Koppula P al (2018) BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 20(10):1181–. https://doi.org/10.1038/s41556-018-0178-0
Jiang L, Kon N, Li TY, Wang SJ, Su T, Hibshoosh H al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–. https://doi.org/10.1038/nature14344
Chu B, Kon N, Chen DL, Li TY, Liu T, Jiang L al (2019) ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 21(5):579–. https://doi.org/10.1038/s41556-019-0305-6
Wang X, Chen Y, Wang X, Tian H, Wang Y, Jin J (2021) Stem cell factor SOX2 confers ferroptosis resistance in lung cancer via upregulation of SLC7A11. Cancer Res 81(20):5217–5229. https://doi.org/10.1158/0008-5472.CAN-21-0567
Wu M, Zhang X, Zhang W, Chiou YS, Qian W, Liu X al (2022) Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nat Commun 13(1):1371. https://doi.org/10.1038/s41467-022-29018-9
Sharbeen G, McCarroll JA, Akerman A, Kopecky C, Youkhana J, Kokkinos J et al (2021) Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma determine response to SLC7A11 inhibition. Cancer Res 81(13):3461–3479. https://doi.org/10.1158/0008-5472.CAN-20-2496
Shimada K, Hayano M, Pagano NC, Stockwell BR (2016) Cell-Line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chem Biology 23(2):225–235. https://doi.org/10.1016/j.chembiol.2015.11.016
Ding CKC, Rose J, Sun TA, Wu JL, Chen PH, Lin CC et al (2020) MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nat Metabolism 2(3):270. https://doi.org/10.1038/s42255-020-0181-1
Lin CCE, Ding CKC, Sun TA, Wu JL, Chen KY, Zhou P, al, (2021) The regulation of ferroptosis by MESH1 through the activation of the integrative stress response. Cell Death Dis. https://doi.org/10.1038/s41419-021-04018-7
Elguindy MM, Nakamaru-Ogiso E (2015) Apoptosis-inducing Factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH: ubiquinone oxidoreductases (NDH-2). J Biol Chem 290(34):20815–20826. https://doi.org/10.1074/jbc.M115.641498
Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ et al (2016) Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 12(7):497. https://doi.org/10.1038/Nchembio.2079
Koppula P, Lei G, Zhang Y, Yan Y, Mao C, Kondiparthi L al (2022) A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat Commun 13(1):2206. https://doi.org/10.1038/s41467-022-29905-1
Mishima E, Ito J, Wu Z, Nakamura T, Wahida A, Doll S al (2022) A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. https://doi.org/10.1038/s41586-022-05022-3
Gao MH, Yi JM, Zhu JJ, Minikes AM, Monian P, Thompson CB et al (2019) Role of mitochondria in ferroptosis. Mol Cell 73(2):354. https://doi.org/10.1016/j.molcel.2018.10.042
Zhang L, Zhou F, van Laar T, Zhang J, van Dam H, Ten Dijke P (2011) Fas-associated factor 1 antagonizes Wnt signaling by promoting beta-catenin degradation. Mol Biol Cell 22(9):1617–1624. https://doi.org/10.1091/mbc.E10-12-0985
Zhang L, Zhou F, Li Y, Drabsch Y, Zhang J, van Dam H et al (2012) Fas-associated factor 1 is a scaffold protein that promotes beta-transducin repeat-containing protein (beta-TrCP)-mediated beta-catenin ubiquitination and degradation. J Biol Chem 287(36):30701–30710. https://doi.org/10.1074/jbc.M112.353524
Kim H, Rodriguez-Navas C, Kollipara RK, Kapur P, Pedrosa I, Brugarolas J (2015) Unsaturated fatty acids stimulate tumor growth through stabilization of beta-catenin. Cell Rep 13(3):495–503. https://doi.org/10.1016/j.celrep.2015.09.010
Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F al (2020) Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16(12):1351–. https://doi.org/10.1038/s41589-020-0613-y
Roh JL, Kim EH, Jang H, Shin D (2017) Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol 11:254–262. https://doi.org/10.1016/j.redox.2016.12.010
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107. https://doi.org/10.1016/j.redox.2019.101107
Shin D, Kim EH, Lee J, Roh JL (2018) Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med 129:454–462. https://doi.org/10.1016/j.freeradbiomed.2018.10.426
Anandhan A, Dodson M, Schmidlin CJ, Liu P, Zhang DD (2020) Breakdown of an ironclad defense system: the critical role of NRF2 in mediating ferroptosis. Cell Chem Biol 27(4):436–447. https://doi.org/10.1016/j.chembiol.2020.03.011
Wu KC, Cui JY, Klaassen CD (2011) Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123(2):590–600. https://doi.org/10.1093/toxsci/kfr183
Song X, Long D (2020) Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front Neurosci 14:267. https://doi.org/10.3389/fnins.2020.00267
Kerins MJ, Ooi A (2018) The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Sign 29(17):1756–1773. https://doi.org/10.1089/ars.2017.7176
Chen D, Chu B, Yang X, Liu Z, Jin Y, Kon N (2021) iPLA2β-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat Commun 12(1):1–15
Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U al (2016) Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol 9:22–31. https://doi.org/10.1016/j.redox.2016.05.003
Llabani E, Hicklin RW, Lee HY, Motika SE, Crawford LA, Weerapana E et al (2019) Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat Chem 11(6):521–532. https://doi.org/10.1038/s41557-019-0261-6
Jelinek A, Heyder L, Daude M, Plessner M, Krippner S, Grosse R al (2018) Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med 117:45–57. https://doi.org/10.1016/j.freeradbiomed.2018.01.019
Butler LM, Perone Y, Dehairs J, Lupien LE, de Laat V, Talebi A (2020) Lipids and cancer: emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv Drug Deliv Rev 159:245–293. https://doi.org/10.1016/j.addr.2020.07.013
Xu H, Chen Y, Gu M, Liu C, Chen Q, Zhan M (2021) Fatty acid metabolism reprogramming in advanced prostate cancer. Metabolites. https://doi.org/10.3390/metabo11110765
Hao Y, Li D, Xu Y, Ouyang J, Wang Y, Zhang Y al (2019) Investigation of lipid metabolism dysregulation and the effects on immune microenvironments in pan-cancer using multiple omics data. BMC Bioinformatics 20(Suppl 7):195. https://doi.org/10.1186/s12859-019-2734-4
Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr (2013) Cellular fatty acid metabolism and cancer. Cell Metab 18(2):153–161. https://doi.org/10.1016/j.cmet.2013.05.017
Su X, Abumrad NA (2009) Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab 20(2):72–77. https://doi.org/10.1016/j.tem.2008.11.001
Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z (2021) Lipid metabolism and cancer. J Exp Med. https://doi.org/10.1084/jem.20201606
Gallagher EJ, Zelenko Z, Neel BA, Antoniou IM, Rajan L, Kase N et al (2017) Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene 36(46):6462–6471. https://doi.org/10.1038/onc.2017.247
Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O al (2015) Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. P Natl Acad Sci USA 112(8):2473–2478. https://doi.org/10.1073/pnas.1421601112
Zhou T, Zhan JH, Fang WF, Zhao YY, Yang YP, Hou X (2017) Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC). BMC Cancer. https://doi.org/10.1186/s12885-017-3239-z
Rink JS, Lin AY, McMahon KM, Calvert AE, Yang S, Taxter T et al (2021) Targeted reduction of cholesterol uptake in cholesterol-addicted lymphoma cells blocks turnover of oxidized lipids to cause ferroptosis. J Biol Chem 296:100100. https://doi.org/10.1074/jbc.RA120.014888
Liu W, Chakraborty B, Safi R, Kazmin D, Chang CY, McDonnell DP (2021) Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun 12(1):5103. https://doi.org/10.1038/s41467-021-25354-4
Khwairakpam AD, Banik K, Girisa S, Shabnam B, Shakibaei M, Fan L al (2020) The vital role of ATP citrate lyase in chronic diseases. J Mol Med 98(1):71–95
Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L al (2020) Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol 22(2):225–234. https://doi.org/10.1038/s41556-020-0461-8
Ning Z, Guo X, Liu X, Lu C, Wang A, Wang X al (2022) USP22 regulates lipidome accumulation by stabilizing PPARgamma in hepatocellular carcinoma. Nat Commun 13(1):2187. https://doi.org/10.1038/s41467-022-29846-9
Collins JM, Neville MJ, Hoppa MB, Frayn KN (2010) De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem 285(9):6044–6052. https://doi.org/10.1074/jbc.M109.053280
Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW et al (2020) Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A 117(51):32433–32442. https://doi.org/10.1073/pnas.2006828117
Peck B, Schulze A (2016) Lipid desaturation - the next step in targeting lipogenesis in cancer? FEBS J 283(15):2767–2778. https://doi.org/10.1111/febs.13681
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z (2020) HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep 33(10):108487. https://doi.org/10.1016/j.celrep.2020.108487
Yang J, Wang LH, Jia RB (2020) Role of de novo cholesterol synthesis enzymes in cancer. J Cancer 11(7):1761–1767. https://doi.org/10.7150/jca.38598
Jun SY, Brown AJ, Chua NK, Yoon JY, Lee JJ, Yang JO et al (2021) Reduction of squalene epoxidase by cholesterol accumulation accelerates colorectal cancer progression and metastasis. Gastroenterology 160(4):1194. https://doi.org/10.1053/j.gastro.2020.09.009
Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R al (2019) Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567(7746):118–122. https://doi.org/10.1038/s41586-019-0945-5
Zhang N, Zhang HW, Liu Y, Su P, Zhang JS, Wang XL et al (2019) SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with Snail and HDAC1/2. Cell Death Differ 26(5):843–859. https://doi.org/10.1038/s41418-018-0158-8
Cheng CM, Geng F, Li Z, Zhong YG, Wang HB, Cheng X al (2022) Ammonia stimulates SCAP/Insig dissociation and SREBP-1 activation to promote lipogenesis and tumour growth. Nat Metabolism. https://doi.org/10.1038/s42255-022-00568-y
Carracedo A, Cantley LC, Pandolfi PP (2013) Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 13(4):227–232. https://doi.org/10.1038/nrc3483
Wang C, Shao L, Pan C, Ye J, Ding Z, Wu J al (2019) Elevated level of mitochondrial reactive oxygen species via fatty acid beta-oxidation in cancer stem cells promotes cancer metastasis by inducing epithelial-mesenchymal transition. Stem Cell Res Ther 10(1):175. https://doi.org/10.1186/s13287-019-1265-2
Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y al (2022) Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun 13(1):1511. https://doi.org/10.1038/s41467-022-29137-3
Miess H, Dankworth B, Gouw AM, Rosenfeldt M, Schmitz W, Jiang M al (2018) The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37(40):5435–5450. https://doi.org/10.1038/s41388-018-0315-z
Geng F, Cheng X, Wu XN, Yoo JY, Cheng CM, Guo JYH et al (2016) Inhibition of SOAT1 Suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin Cancer Res 22(21):5337–5348. https://doi.org/10.1158/1078-0432.Ccr-15-2973
Cheng X, Geng F, Pan MX, Wu XN, Zhong YG, Wang CY et al (2020) Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab 32(2):229. https://doi.org/10.1016/j.cmet.2020.06.002
Olzmann JA, Carvalho P (2019) Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20(3):137–155. https://doi.org/10.1038/s41580-018-0085-z
Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM et al (2014) Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep 9(1):349–365. https://doi.org/10.1016/j.celrep.2014.08.056
Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L al (2021) Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab 33(8):1701–1715e1705. https://doi.org/10.1016/j.cmet.2021.05.016
Mukhopadhyay S, Schlaepfer IR, Bergman BC, Panda PK, Praharaj PP, Naik PP et al (2017) ATG14 facilitated lipophagy in cancer cells induce ER stress mediated mitoptosis through a ROS dependent pathway. Free Radical Bio Med 104:199–213. https://doi.org/10.1016/j.freeradbiomed.2017.01.007
Panda PK, Patra S, Naik PP, Praharaj PP, Mukhopadhyay S, Meher BR et al (2020) Deacetylation of LAMP1 drives lipophagy-dependent generation of free fatty acids by Abrus agglutinin to promote senescence in prostate cancer. J Cell Physiol 235(3):2776–2791. https://doi.org/10.1002/jcp.29182
Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H al (2019) Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun 508(4):997–1003. https://doi.org/10.1016/j.bbrc.2018.12.039
Maan M, Peters JM, Dutta M, Patterson AD (2018) Lipid metabolism and lipophagy in cancer. Biochem Bioph Res Co 504(3):582–589. https://doi.org/10.1016/j.bbrc.2018.02.097
Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF (2010) Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 140(1):49–61. https://doi.org/10.1016/j.cell.2009.11.027
Ding LG, Sun WF, Balaz M, He AY, Klug M, Wieland S et al (2021) Peroxisomal beta-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat Metabolism 3(12):1648. https://doi.org/10.1038/s42255-021-00489-2
Xuan Y, Wang H, Yung MM, Chen F, Chan WS, Chan YS et al (2022) SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics 12(7):3534–3552. https://doi.org/10.7150/thno.70194
Yi J, Zhu J, Wu J, Thompson CB, Jiang X (2020) Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci U S A 117(49):31189–31197. https://doi.org/10.1073/pnas.2017152117
Wang K, Zhang Z, Tsai HI, Liu Y, Gao J, Wang M al (2021) Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ 28(4):1222–1236. https://doi.org/10.1038/s41418-020-00644-4
Li C, Dong X, Du W, Shi X, Chen K, Zhang W al (2020) LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct Target Ther 5(1):187. https://doi.org/10.1038/s41392-020-00297-2
Ma X, Xiao L, Liu L, Ye L, Su P, Bi E al (2021) CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab 33(5):1001–1012e1005. https://doi.org/10.1016/j.cmet.2021.02.015
Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R al (2021) Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity 54(7):1561–1577e1567. https://doi.org/10.1016/j.immuni.2021.05.003
Kamili A, Roslan N, Frost S, Cantrill LC, Wang DW, Della-Franca A et al (2015) TPD52 expression increases neutral lipid storage within cultured cells. J Cell Sci 128(17):3223–3238. https://doi.org/10.1242/jcs.167692
Zechner R, Madeo F, Kratky D (2017) Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18(11):671–684. https://doi.org/10.1038/nrm.2017.76
Liu K, Czaja MJ (2013) Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ 20(1):3–11. https://doi.org/10.1038/cdd.2012.63
Yang MH, Chen P, Liu J, Zhu S, Kroemer GO, Klionsky DN et al (2019) Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. https://doi.org/10.1126/sciadv.aaw2238
Tan SK, Mahmud I, Fontanesi F, Puchowicz M, Neumann CKA, Griswold AJ et al (2021) Obesity-dependent adipokine chemerin suppresses fatty acid oxidation to confer ferroptosis resistance. Cancer Discov 11(8):2072–2093. https://doi.org/10.1158/2159-8290.CD-20-1453
Justus CR, Dong L, Yang LV (2013) Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol 4:354. https://doi.org/10.3389/fphys.2013.00354
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C et al (2021) Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab 33(10):2040-2058 e2010. https://doi.org/10.1016/j.cmet.2021.09.002
Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E al (2019) Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4(+) T Cell Metabolic Rewiring. Cell Metab 30(6):1055–. https://doi.org/10.1016/j.cmet.2019.10.004
Zhang HY, Deng T, Liu R, Ning T, Yang HO, Liu DY et al (2020) CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. https://doi.org/10.1186/s12943-020-01168-8
Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L (2016) Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165(5):1092–1105. https://doi.org/10.1016/j.cell.2016.04.009
Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK et al (2019) CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569(7755):270–274. https://doi.org/10.1038/s41586-019-1170-y
Kim D, Kim WD, Kim SK, Moon DH, Lee SJ (2020) TGF-beta 1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. https://doi.org/10.1038/s41419-020-2618-6
Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond V et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180-U1120. https://doi.org/10.1038/ncb3064
Angeli JPF, Krysko DV, Conrad M (2019) Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer 19(7):405–414
Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S (2018) NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172(5):1022
Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ et al (2021) Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 28(6):1971–1989. https://doi.org/10.1038/s41418-020-00719-2
Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S al (2017) The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun 8(1):864. https://doi.org/10.1038/s41467-017-00910-z
Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R et al (2020) Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol 16(3):278–290. https://doi.org/10.1038/s41589-019-0462-8
Muri J, Thut H, Bornkamm GW, Kopf M (2019) B1 and marginal zone B cells but not follicular B2 cells require Gpx4 to prevent lipid peroxidation and ferroptosis. Cell Rep 29(9):2731-2744e2734. https://doi.org/10.1016/j.celrep.2019.10.070
Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB et al (2020) Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immun Can. 8(1):e000337
Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P (2010) Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Bba-Rev Cancer 1805(1):53–71
Snyder AG, Hubbard NW, Messmer MN, Kofman SB, Hagan CE, Orozco SL et al (2019) Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci Immunol 4(36):eaaw2004
Wen Q, Liu J, Kang R, Zhou B, Tang D (2019) The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 510(2):278–283. https://doi.org/10.1016/j.bbrc.2019.01.090
Turubanova VD, Balalaeva IV, Mishchenko TA, Catanzaro E, Alzeibak R, Peskova NN et al (2019) Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer 7(1):350. https://doi.org/10.1186/s40425-019-0826-3
Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA et al (2020) Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. https://doi.org/10.1136/jitc-2020-001369
Li DS, Li YS (2020) The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Tar. https://doi.org/10.1038/s41392-020-00216-5
Dai EY, Han L, Liu J, Xie YC, Kroemer G, Klionsky DJ et al (2020) Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 16(11):2069–2083. https://doi.org/10.1080/15548627.2020.1714209
Dai EY, Han L, Liu J, Xie YC, Zeh HJ, Kang R et al (2020) Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun. https://doi.org/10.1038/s41467-020-20154-8
Granchi C (2018) ATP citrate lyase (ACLY) inhibitors: an anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem 157:1276–1291. https://doi.org/10.1016/j.ejmech.2018.09.001
Huang ZG, Zhang M, Plec AA, Estill SJ, Cai L, Repa JJ et al (2018) ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. P Natl Acad Sci USA 115(40):E9499–E9506. https://doi.org/10.1073/pnas.1806635115
Miller KD, Pniewski K, Perry CE, Papp SB, Shaffer JD, Velasco-Silva JN et al (2021) Targeting ACSS2 with a transition-state mimetic inhibits triple-negative breast cancer growth. Cancer Res 81(5):1252–1264. https://doi.org/10.1158/0008-5472.Can-20-1847
Lally JSV, Ghoshal S, DePeralta DK, Moaven O, Wei L, Masia R et al (2019) Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab 29(1):174. https://doi.org/10.1016/j.cmet.2018.08.020
Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN et al (2016) Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med 22(10):1108–1119. https://doi.org/10.1038/nm.4181
Gouw AM, Margulis K, Liu NS, Raman SJ, Mancuso A, Toal GG et al (2019) The MYC oncogene cooperates with sterol-regulated element-binding protein to regulate lipogenesis essential for neoplastic growth. Cell Metab 30(3):556. https://doi.org/10.1016/j.cmet.2019.07.012
Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS et al (2015) Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27(1):57–71. https://doi.org/10.1016/j.ccell.2014.12.002
Menendez JA, Lupu R (2017) Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin Ther Tar 21(11):1001–1016. https://doi.org/10.1080/14728222.2017.1381087
Zaytseva YY, Rychahou PG, Le AT, Scott TL, Flight RM, Kim JT et al (2018) Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget 9(37):24787–24800. https://doi.org/10.18632/oncotarget.25361
Heuer TS, Ventura R, Mordec K, Lai J, Fridlib M, Buckley D (2017) FASN inhibition and taxane treatment combine to enhance anti-tumor efficacy in diverse xenograft tumor models through disruption of tubulin palmitoylation and microtubule organization and FASN inhibition-mediated effects on oncogenic signaling and gene expression. Ebiomedicine 16:51–62
Kim S, Kang WK, Lee J, Park SH, Park JO, Park YS et al (2014) Simvastatin plus capecitabine-cisplatin (XP) versus placebo plus capecitabine-cisplatin (XP) in patients with previously untreated advanced gastric cancer: a double-blind randomized phase 3 study. J Clin Oncol. https://doi.org/10.1200/jco.2014.32.15_suppl.4066
Perez MA, Magtanong L, Dixon SJ, Watts JL (2020) Dietary lipids induce ferroptosis in caenorhabditis elegans and human cancer cells. Dev Cell 54(4):447. https://doi.org/10.1016/j.devcel.2020.06.019
Fang X, Xu Y, Wang S, Wan J, He C, Chen M (2017) Pluronic F68-Linoleic acid nano-spheres mediated delivery of gambogic acid for cancer therapy. AAPS PharmSciTech 18(1):147–155. https://doi.org/10.1208/s12249-015-0473-z
Berquin IM, Edwards IJ, Chen YQ (2008) Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett 269(2):363–377. https://doi.org/10.1016/j.canlet.2008.03.044
Wei LY, Wu ZP, Chen YQ (2022) Multi-targeted therapy of cancer by omega-3 fatty acids-an update. Cancer Lett 526:193–204. https://doi.org/10.1016/j.canlet.2021.11.023
Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L (2019) Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov 9(12):1673–1685. https://doi.org/10.1158/2159-8290.CD-19-0338
Lei G, Mao C, Yan Y, Zhuang L, Gan B (2021) Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 12(11):836–857. https://doi.org/10.1007/s13238-021-00841-y
Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH et al (2020) The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res 30(2):146–162. https://doi.org/10.1038/s41422-019-0263-3
Lovey J, Nie D, Tovari J, Kenessey I, Timar J, Kandouz M al (2013) Radiosensitivity of human prostate cancer cells can be modulated by inhibition of 12-lipoxygenase. Cancer Lett 335(2):495–501. https://doi.org/10.1016/j.canlet.2013.03.012
Padanad MS, Konstantinidou G, Venkateswaran N, Melegari M, Rindhe S, Mitsche M et al (2016) Fatty acid oxidation mediated by Acyl-CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep 16(6):1614–1628. https://doi.org/10.1016/j.celrep.2016.07.009
Liu G, Kuang S, Cao RB, Wang J, Peng QC, Sun CM (2019) Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J 33(9):10089–10103. https://doi.org/10.1096/fj.201802619RR
Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J (2019) Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res 79(20):5355–5366. https://doi.org/10.1158/0008-5472.CAN-19-0369
Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J al (2022) Ferroptosis in cancer and cancer immunotherapy. Cancer Commun (Lond) 42(2):88–116. https://doi.org/10.1002/cac2.12250
Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY et al (2018) Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest 128(8):3341–3355. https://doi.org/10.1172/Jci99032
Weiwer M, Bittker JA, Lewis TA, Shimada K, Yang WS, MacPherson L al (2012) Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg Med Chem Lett 22(4):1822–1826. https://doi.org/10.1016/j.bmcl.2011.09.047
Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, Nicoletti P (2015) Elucidating compound mechanism of action by network perturbation analysis. Cell 162(2):441–451. https://doi.org/10.1016/j.cell.2015.05.056
Gao J, Yang F, Che J, Han Y, Wang Y, Chen N (2018) Selenium-encoded isotopic signature targeted profiling. ACS Cent Sci 4(8):960–970. https://doi.org/10.1021/acscentsci.8b00112
Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC et al (2015) Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27(2):211–222. https://doi.org/10.1016/j.ccell.2014.11.019
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C (2018) Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat 50(2):445–460. https://doi.org/10.4143/crt.2016.572
Lo M, Ling V, Low C, Wang YZ, Gout PW (2010) Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Curr Oncol 17(3):9–16
Yu H, Yang C, Jian L, Guo S, Chen R, Li K (2019) Sulfasalazineinduced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol Rep 42(2):826–838. https://doi.org/10.3892/or.2019.7189
Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Maziere JC, Chauffert B al (2013) Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer 133(7):1732–1742. https://doi.org/10.1002/ijc.28159
Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM et al (2019) Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol 26(5):623-633e629. https://doi.org/10.1016/j.chembiol.2019.01.008
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X al (2015) HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 34(45):5617–5625
Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G al (2017) Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 36(29):4089–4099. https://doi.org/10.1038/onc.2017.11
Gaschler MM, Andia AA, Liu HR, Csuka JM, Hurlocker B, Vaiana CA et al (2018) FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14(5):507. https://doi.org/10.1038/s41589-018-0031-6
Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS et al (2020) Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol 15(2):469–484. https://doi.org/10.1021/acschembio.9b00939
Xu S, Min JX, Wang FD (2021) Ferroptosis: an emerging player in immune cells. Sci Bull 66(22):2257–2260. https://doi.org/10.1016/j.scib.2021.02.026
Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G al (2021) Lipid signalling enforces functional specialization of Treg cells in tumours. Nature 591(7849):306–311. https://doi.org/10.1038/s41586-021-03235-6
Liu X, Hartman CL, Li L, Albert CJ, Si F, Gao A et al (2021) Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaz6314
Basit F, van Oppen LM, Schockel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC et al (2017) Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis 8(3):e2716. https://doi.org/10.1038/cddis.2017.133
Acknowledgements
The graphical abstracts were created with BioRender software (BioRender.com).−
Funding
This work was supported by the National Natural Science Foundation of China (81972479, U2004118 and 82072899), Natural Science Foundation of Guangdong province (2019A1515011100 and 2021A1515012576), Henan Natural Science Foundation (202300410359) and Henan Medical Research Program (SBGJ2020002081), Guangzhou High-Level Clinical Key Specialty Construction Project; Clinical Key Specialty Construction Project of Guangzhou Medical University (202005), the Innovation Project of Universities in Guangdong Province (No. 2021KTSCX026), Scientific and Technological Planning Project of Guangzhou City (No. 201904010038), Special project of South China Normal University for foreign exchange in Guangdong Hong Kong Macao Great Bay Area in 2022.
Author information
Authors and Affiliations
Contributions
ZZ and ML conceived the study and drafted de manuscript. JY, HL, HL and XH collected the related references. ZZ, YC and QL revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agreed with the content of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, M., Yan, J., Hu, X. et al. Targeting lipid metabolism for ferroptotic cancer therapy. Apoptosis 28, 81–107 (2023). https://doi.org/10.1007/s10495-022-01795-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01795-0
Keywords
Profiles
- Zhengzhi Zou View author profile