Abstract
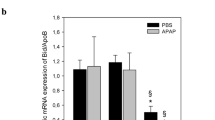
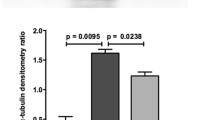
Acute liver failure (ALF) is a life threatening disease for which only few treatment options exist. The molecular pathways of disease progression are not well defined, but the death receptor Fas (CD95/Apo-1) appears to play a pivotal role in hepatocyte cell death and the development of ALF. Here, we explored posttranscriptional gene silencing of Fas by RNAi to inhibit pathophysiological gene expression. For targeting Fas expression in mice, Fas siRNA was formulated with the liver-specific siRNA delivery system DBTC. Treatment of mice with DBTC/siRNAFas reduced Fas expression in the liver, but not in the spleen, lung, kidney or heart. Furthermore, silencing of Fas receptor was effective in blocking or reducing several aspects of ALF when it was tested in mice exposed to galactosamine/lipopolysaccharide (G/L), a well-known model of ALF. The application of DBTC/siRNAFas 48 h prior G/L exposure resulted in amelioration of hepatic perfusion, reduction of hepatocellular death and increase of survival rate. The administration of DBTC/siRNAFas formulation further diminished the inflammatory response upon G/L challenge, as indicated by a marked decrease of TNFα mRNA expression. However, IL-6 plasma concentration remained unaffectedly by DBTC/siRNAFas formulation. Since the specific silencing of hepatic Fas expression only partially protected from inflammation, but completely attenuated apoptotic and necrotic cell death as well as microcirculatory dysfunction, the development of therapeutic strategies with DBTC lipoplex formulations to treat ALF should be combined with anti-inflammatory strategies to reach maximal therapeutic efficacy.







Similar content being viewed by others
References
Antoniades CG, Berry PA, Wendon JA, Vergani D (2008) The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol 49:845–861
Faubion WA, Gores GJ (1999) Death receptors in liver biology and pathobiology. Hepatology 29:1–4
Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L (1995) Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med 182:1223–1230
Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178
Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S (1997) Essential roles of the Fas ligand in the development of hepatitis. Nat Med 3:409–413
Ksontini R, Colagiovanni DB, Josephs MD, Edwards CK 3rd, Tannahill CL, Solorzano CC, Norman J, Denham W, Clare-Salzler M, MacKay SL, Moldawer LL (1998) Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J Immunol 160:4082–4089
Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J (2003) RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 9:347–351
Hannon GJ (2002) RNA interference. Nature 418:244–251
Jiang N, Zhang X, Zheng X, Chen D, Siu K, Wang H, Ichim TE, Quan D, McAlister V, Chen G, Min WP (2012) A novel in vivo siRNA delivery system specifically targeting liver cells for protection of ConA-induced fulminant hepatitis. PLoS One 7:e44138
Le Minh K, Klemm K, Abshagen K, Eipel C, Menger MD, Vollmar B (2007) Attenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of mice. Am J Pathol 170:1954–1963
Eipel C, Kidess E, Abshagen K, Leminh K, Menger MD, Burkhardt H, Vollmar B (2007) Antileukoproteinase protects against hepatic inflammation, but not apoptosis in the response of D-galactosamine-sensitized mice to lipopolysaccharide. Br J Pharmacol 151:406–413
Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang GZ, Takahashi K, Tamada Y, Yokochi T (1996) Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun 64:734–738
Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A (1995) Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 146:1220–1234
Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, Löffler K, Fechtner M, Röhl T, Fisch G, Dames S, Arnold W, Giese K, Klippel A, Kaufmann J (2006) RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther 13:1360–1370
Czauderna F, Fechtner M, Dames S, Aygün H, Klippel A, Pronk GJ, Giese K, Kaufmann J (2003) Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res 31:2705–2716
Schäfer T, Scheuer C, Roemer K, Menger MD, Vollmar B (2003) Inhibition of p53 protects liver tissue against endotoxin-induced apoptotic and necrotic cell death. FASEB J 17:660–667
Fehring V, Schaeper U, Ahrens K, Santel A, Keil O, Eisermann M, Giese K, Kaufmann J (2014) Delivery of therapeutic siRNA to the lung endothelium via novel Lipoplex formulation DACC. Mol Ther 22:811–820
Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456
Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH (1999) CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J Exp Med 189:441–446
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S (1993) Lethal effect of the anti-Fas antibody in mice. Nature 364:806–809
Kuhla A, Eipel C, Siebert N, Abshagen K, Menger MD, Vollmar B (2008) Hepatocellular apoptosis is mediated by TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of acute liver failure. Apoptosis 13:1427–1438
Karikó K, Bhuyan P, Capodici J, Weissman D (2004) Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol 172:6545–6549
Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A (2005) In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 106:2295–2301
Li X, Zhang JF, Lu MQ, Yang Y, Xu C, Li H, Wang GS, Cai CJ, Chen GH (2007) Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbecks Arch Surg 392:345–351
Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D (2004) Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther 11:675–682
Jaeschke H, Gujral JS, Bajt ML (2004) Apoptosis and necrosis in liver disease. Liver Int 24:85–89
Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R (1988) Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet 2:72–74
Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M (2005) Apoptosis in liver diseases—detection and therapeutic applications. Med Sci Monit 11:RA337–RA345
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43:S31–S44
Han LH, Sun WS, Ma CH, Zhang LN, Liu SX, Zhang Q, Gao LF, Chen YH (2002) Detection of soluble TRAIL in HBV infected patients and its clinical implications. World J Gastroenterol 8:1077–1080
Hu WH, Johnson H, Shu HB (2000) Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem 275:10838–10844
Ahn JH, Park SM, Cho HS, Lee MS, Yoon JB, Vilcek J, Lee TH (2001) Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-kappaB-dependent gene expression. J Biol Chem 276:47100–47106
Wajant H, Pfizenmaier K, Scheurich P (2003) Non-apoptotic Fas signaling. Cytokine Growth Factor Rev 14:53–66
McDonald B, Jenne CN, Zhuo L, Kimata K, Kubes P (2013) Kupffer cells and activation of endothelial TLR4 coordinate neutrophil adhesion within liver sinusoids during endotoxemia. Am J Physiol Gastrointest Liver Physiol 305:G797–G806
Acknowledgments
The authors cordially thank Berit Blendow, Dorothea Frenz, Maren Nerowski, and Eva Lorbeer-Rehfeldt (Institute for Experimental Surgery, University of Rostock, Germany) for excellent technical assistance.
Conflict of interest
Volker Fehring, Ute Schaeper, and Ulf Schulze-Topphoff are employees of and have stock options from Silence Therapeutics plc and declare competing financial interest. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kuhla, A., Thrum, M., Schaeper, U. et al. Liver-specific Fas silencing prevents galactosamine/lipopolysaccharide-induced liver injury. Apoptosis 20, 500–511 (2015). https://doi.org/10.1007/s10495-015-1088-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1088-2