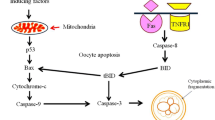
Abstract
We studied the alterations of dying oocytes in 1–28 days old rats using TUNEL method, immunolocalizations of active caspase 3, lamp1, localization of acid phosphatase, and DAPI staining. All procedures were performed in adjacent sections of each oocyte. In most dying oocytes exist simultaneously features of apoptosis as active caspase 3 and DNA breaks, and a large increase of lamp1 and acid phosphatase characteristic of autophagy. Large clumps of compact chromatin and membrane blebbing were absent. Electron microscope observations demonstrated the presence of small clear vesicles and autophagolysosomes. All these features indicate that a large number of oocytes are eliminated by a process sharing features of apoptosis and autophagy. In dying oocytes of new born rats the markers of apoptosis predominate over those of autophagy. However, fragmentation and apoptotic bodies were not found. These features suggest that in different cytophysiological conditions the processes of cell death may be differently modulated.
















Similar content being viewed by others
References
Hirshfield AN (1991) Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–101. doi:10.1016/S0074-7696(08)61524-7
Morita Y, Tilly JL (1999) Oocyte apoptosis: like sand through an hourglass. Dev Biol 213:1–17. doi:10.1006/dbio.1999.9344
Tilly JL, Robles R (1999) Apoptosis and its impact in clinical reproductive medicine. In: Molecular biology in reproductive medicine. Parthenon, New York, pp 79–101
Murdoch WJ (2000) Proteolytic and cellular death mechanisms in ovulatory ovarian rupture. Biol Sings Recept 9:102–114
Rueda BR, Hoyer PB, Hamemlk DL, Tilly JL (1997) Potential regulators of physiological cell death in the corpus luteum. In: Cell death in reproductive physiology. Springer-Verlag, New York, pp 161–181
Garrett WM, Guthrie HD (1999) Expression of Bcl-2 and 3-hydroxysteroid dehydrogenase protein during oocyte and follicle development in foetal and post-natal pig ovaries. Reprod Fertil Dev 11:463–470. doi:10.1071/RD00003
Kerr JFR, Willie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics B. J Cancer 26:239–257
Baehrecke EH (2005) Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 6:505–510. doi:10.1038/nrm1666
Aki T, Yamagychi K, Fujimiya T, Mizukami Y (2003) Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene 22:8529–8535
Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, Walker R, Hermann RS (1996) Active cell death induced by the antiestrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis 17:1595–1607. doi:10.1093/carcin/17.8.1595
Schweichel JU, Merker HJ (1973) The morphology of various types of cell death in prenatal tissues. Teratology 7:253–266. doi:10.1002/tera.1420070306
Clarke PGH (1990) Development cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 181:195–213. doi:10.1007/BF00174615
Gozuacik D, Kimchi A (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23:2891–2906. doi:10.1038/sj.onc.1207521
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477. doi:10.1016/S1534-5807(04)00099-1
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306:990–995. doi:10.1126/science.1099993
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721. doi:10.1126/science.290.5497.1717
Lu Y, Lenardo MJ, Baechrecke EH (2004) Autophagy and caspases: a new cell death program. Cell Cycle 3:1124–1126
Bursch W (2001) The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 8:569–581. doi:10.1038/sj.cdd.4400852
Fukuda M (1991) Lysosomal membrane glycoproteins. Structure, biosynthesis and intracellular trafficking. J Biol Chem 266:21327
Sawada R, Jardine KA, Fukuda M (1993) The genes of major lysosomal membrane glycoproteins lamp1 and lamp2. The 5′-flanking sequence of lamp2 gene and comparison of exon organization in two genes. J Biol Chem 268:13010
Perez GI, Tao XJ, Tilly JL (1999) Fragmentation and death (a. k. a. apoptosis) of ovulated oocytes. Mol Hum Reprod 5:414–420. doi:10.1093/molehr/5.5.414
Perez GI, Jurisicova A, Matikainen T, Moriyama T, Kim MR, Takai Y et al (2005) A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J 19:860–862
Ortiz R, Echeverría OM, Salgado R, Escobar ML, Vázquez-Nin GH (2006) Fine structural and cytochemical analysis of the processes of cell death of oocytes in atretic follicles in new born and prepubertal rats. Apoptosis 11:25–37. doi:10.1007/s10495-005-3347-0
Guide Line for the Care and Use of Laboratory Animals (1996) The National Academies Press, Washington, DC, pp 1–140
Gomori G (1950) An improved histochemical technic for acid phosphatase. Stain Technol 25:81
Chen P, Abrams JM (2000) Drosophila apoptosis and Bcl-2 genes: outliers fly in. J Cell Biol 148:625–627
Jolly PD, Smith PR, Heath DA, Hudson NL, Lun S, Still LA et al (1997) Morphological evidence of apoptosis and prevalence of apoptotic versus mitotic cells in the membrane granulose of ovarian follicles during spontaneous and induced atresia in ewes. Biol Reprod 56:837–846. doi:10.1095/biolreprod56.4.837
Hughes FM Jr, Gecospe WC (1991) Biochemical identification of apoptosis (programmed cell death) in granulos cells. Evidence form a potencial mechanism undrerlying follicular atresia. Endocrinology 129:2415–2422
Tilly JL (1997) Apoptosis and the ovary: a fashionable trend of food for thought? Fertil Steril 67:226–228. doi:10.1016/S0015-0282(97)81901-2
Fenwick MA, Hurse PR (2002) Inmmunohistochemical localization of active caspase 3 in the mouse ovary. Growth and atresia of small follicles. Reproduction 124:659–663. doi:10.1530/rep.0.1240659
Matilkainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA et al (2001) Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology 142:2468–2480. doi:10.1210/en.142.6.2468
Quirk SM, Cowan RG, Harman RM (2005) Progesterone receptor and the cell cycle modulate apoptosis in granulose cells. Endocrinology 145:5033–5043. doi:10.1210/en.2004-0140
D’Herde K, De Prest B, Roels F (1996) Subtypes of active cell death in the granulosa of ovarian atretic follicles in the quail (Coturnix coturnix japónica). Reprod Nutr Dev 36:175–189. doi:10.1051/rnd:19960203
Kovacs J, Forgo V, Peczely P (1992) The fine structure of the follicular cells of the domestic goose. Cell Tissue Res 267:561–569. doi:10.1007/BF00319379
Takeda M, Shirato I, Kobayashi M, Endou H (1999) Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron 81:234–238. doi:10.1159/000045282
Martin DH, Baehrecke AH (2004) Caspase function in autophagic cell death in Drosophila. Development 131:275–284
Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040. doi:10.1128/MCB.25.3.1025-1040.2005
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141. doi:10.1016/S0300-9084(02)01369-X
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889. doi:10.1038/nature04724
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15:171–182. doi:10.1038/sj.cdd.4402233
Lee CY, Clough EA, Yellon P, Teslovich TM, Stephan DA, Bachrecke EHN (2003) Genome-wide analysis of steroid an aradiation-triggered programmed cell death in Drosophila. Curr Biol 13:350–357
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752. doi:10.1038/nrm2239
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L et al (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of atg4. EMBO J 26:1749–1760. doi:10.1038/sj.emboj.7601623
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al (2004) Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 6:1221–1228. doi:10.1038/ncb1192
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N et al (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122(6):927–939. doi:10.1016/j.cell.2005.07.002
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T et al (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25:193–205. doi:10.1016/j.molcel.2006.12.009
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676
Yue Z, Jin S, Yang C, Levine AJ, Heintz N (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 100:15077–15082. doi:10.1073/pnas.2436255100
Yoshimori T (2004) Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun 313:453–458. doi:10.1016/j.bbrc.2003.07.023
Shunsuke K, Takeshi N, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein tandem fluorescent-tagged LC3. Autophagy 3(5):452–460
Carmo-Fonseca M, Mendes-Suares L, Campos I (2000) To be or not to be in the nucleolus. Nat Cell Biol 2:E107–E112. doi:10.1038/35014078
Lam YW, Trinkle-Mulcahy L, Lamond AI (2005) The nucleolus. J Cell Sci 118:1335–1337. doi:10.1242/jcs.01736
Sirri V, Urcuqui-Inchima S, Roussel P, Hernandez-Verdun D (2008) Nucleolus: the fascinating nuclear body. Histochem Cell Biol 129(1):13–31. doi:10.1007/s00418-007-0359-6
Visintin R, Amon A (2000) The nucleolus: the magician’s hat for cell cycle tricks. Curr Opin Cell Biol 12:372–377. doi:10.1016/S0955-0674(00)00102-2
Acknowledgments
PAPIIT IN211905-3, PAPIIT IN203308-3, CONACYT 36450-N. We thank Silvia Juárez and Ernestina Ubaldo for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escobar, M.L., Echeverría, O.M., Ortíz, R. et al. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis 13, 1253–1266 (2008). https://doi.org/10.1007/s10495-008-0248-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0248-z