Abstract
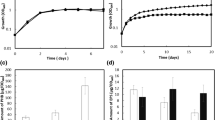
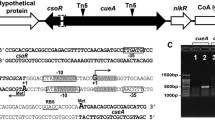
The aim of this investigation was to identify and isolate genes involved in acid tolerance from Sinorhizobium sp. strain BL3. It was hypothesized that acid tolerance of strain BL3 could be enhanced by high level expression of certain genes involved in acid tolerance, following insertion of these genes in a multiple copy plasmid. A cosmid clone library of BL3 was introduced into BL3, and the transconjugant colonies were selected at low pH. A single cosmid containing genes for acid tolerance was isolated from 40 different colonies. By transposon–insertion mutagenesis, subcloning and DNA sequencing, a gene involved in acid tolerance, actX, was identified in a 4.4-kb fragment of this cosmid. The actX mutant of BL3 showed increased acid sensitivity and was complemented by the 4.4-kb subcloned fragment. Phaseolus lathyroides seedlings inoculated with recombinant strains containing multiple copies of actX showed increased symbiotic performance at low pH. By constructing an actX::gus fusion, it was shown that actX was induced at low pH. actX encodes a putative histidine kinase sensor protein of a two-component regulatory system. The method of gene identification used in this study for isolation of actX may be applied for the isolation of other genes involved in tolerance to adverse environmental factors.
Similar content being viewed by others
References
Beringer J.E., Beynon J., Buchanan-Wollaston A.V., Johnston A.W.B. (1978). Transfer of the drug resistance transposon Tn5 to Rhizobium. Nature. 276:633–634
Bothakur D., Soedarjo M., Fox P.M., Webb D.T. (2003). The mid genes of Rhizobium sp. strain TAL1145 are required for degradation of mimosine into 3-hydroxy-4-pyridone and are inducible by mimosine. Microbiology 149:537–546
Chen J., Richardson A.E., Rolfe B.J. (1993). Studies on the physiological and genetic basis of acid tolerance in Rhizobium leguminosarum biovar trifolii. Appl. Environ. Microbiol. 59:1798–1804
Dilworth M.J., Glenn A.R. (1999). Problems of adverse pH and bacterial strategies to combat it. In: Chadwick DJ, Gardew G. (eds), Bacterial responses to pH. England, John Wiley and Sons Ltd, pp. 4–18. ISBN 0471985996
Dilworth M.J., Howieson J.G., Reeve W.G., Tiwari R.P., Glenn A.R. (2001). Acid tolerance in legume root nodule bacteria and selecting for it. Aust. J. Exp. Agric. 41:435–446
Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X.W., Finlay D.R., Guiney D., Helinski D.R. (1985). Plasmids related to the broad host-range vector, pRK 290, useful for gene cloning and monitoring gene expression. Plasmid 13:149–153
Figurski D.H., Helinski D.R. (1979). Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Nat. Acad. Sci. USA. 76:1648–1652
Finan T.M., Weidner S., Wong K., Buhrmester J., Chain P., Vorholter F.J., Hernandez-Lucas I., Becker A., Cowie A., Gouzy J., Golding B., Puhler A. (2001). The complete sequence of the 1,683-kb pSymB megaplasmid from the N2-fixing endosymbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. U.S.A. 98:9889–9894
Foster J.W. (1991). Salmonella acid shock proteins are required for the acid tolerance response. J. Bacteriol. 173:6896–6902
Foster J.W., Hall M.K. (1990). Adaptive acidification tolerance response of Salmonella typhimurium. J. Bacteriol. 172:771–778
Fox P.M., Borthakur D. (2001). Selection of several classes of mimosine- degradation-defective Tn3Hogus-insertion mutants of Rhizobium sp. strain TAL1145 on the basis of mimosine-inducible GUS activity. Can. J. Microbiol. 47:488–494
Goodson M., Rowbury R.J. (1989). Habituation to normally lethal acidity by prior growth of Escherichia coli at a sublethal acid pH value. Letts. Appl. Microbiol. 8:77–79
Goss T.J., O’Hara G.W., Dilworth M.J., Glenn A.R. (1990). Cloning, characterization and complementation of lesions causing acid-sensitivity in Tn5-induced mutants of Rhizobium meliloti WSM419. J. Bacteriol. 172: 5173–5179
Howieson J.G. (1985). Use of an organic buffer for the selection of the acid tolerant Rhizobium meliloti strains. Plant Soil. 88:367–376
Johnston A.W.B., Beynon J.L., Buchanan-Wollaston A.V., Setchell S.M., Hirsch P.R., Beringer J.E. (1978). High frequency transfer of nodulating ability between strains and species of Rhizobium. Nature 276:634–636
Kaneko T., Nakamura Y., Sato S., Asamizu E., Kato T., Sasamoto S., Watanabe A., Idesawa K., Ishikawa A., Kawashima K., Kimura T., Kishida Y., Kiyokawa C., Kohara M., Matsumoto M., Matsuno A., Mochizuki Y., Nakayama S., Nakazaki N., Shimpo S., Sugimoto M., Takeuchi C., Yamada M., Tabata S. (2000). Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7: 331–338
Maniatis T., Fritsch E.F., Sambrook J. (1982). Molecular cloning: a laboratory manual Cold Spring Harbor Laboratory. New York, Cold Spring Harbor
Mavingui P., Flores M., Romero D., Martinez-Romero E., Palacios R. (1997). Generation of Rhizobium strains with improved symbiotic properties by random DNA amplification (RDA). Nat. Biotechnol. 15:564–569
O’Hara G.W., Glenn A.R. (1994). The adaptive acid tolerance response in root nodule bacteria and Escherichia coli. Arch. Microbiol. 161:286–292
O’Hara G.W., Goss T.J., Dilworth M.J., Glenn A.R. (1989). Maintenance of intracellular pH and acid tolerance in Rhizobium meliloti. Appl. Environ. Microbiol. 55:1870–1876
Reeve W.G., Tiwari R.P., Kale N.B., Dilworth M.J., Glenn A.R. (2002). ActP controls copper homeostasis in Rhizobium leguminosarum bv. viciae and Sinorhizobium meliloti preventing low pH-induced copper toxicity. Mol. Microbiol. 43:981–991
Reeve W.G., Tiwari R.P., Wong C.M., Dilworth M.J., Glenn A.R. (1998). The transcriptional regulator gene phrR in Sinorhizobium meliloti WSM419 is regulated by low pH and other stresses. Microbiology 144:3335–3342
Reeve W.G., Tiwai R.P., Worsley P.S., Dilworth M.J., Glenn A.R., Howieson J.G. (1999). Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies. Microbiology 145:1507–1516
Ruvkun G.B., Ausubel F.M. (1981). A general method for site-directed mutagenesis in prokaryotes. Nature 289:85–89
Slavik J. (1982). Intracellular pH of yeast cells measured with fluorescent probes. FEBS Letts. 140:22–26
Somasegaran P., Hoben H.J. (1994). Handbook for rhizobia, methods in legume-rhizobium technology. New York, Springer-Verlag New York. Inc. ISBN 0387941347
Staskawicz B., Dahlbeck D., Keen N.T., Napoli C. (1987). Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 169:5789–5794
Tiwari R.P., Reeve W.G., Glenn A.R. (1992). Mutations conferring acid sensitivity in the acid-tolerant strains Rhizobium meliloti WSM419 and Rhizobium leguminosarum biovar viciae WSM710. FEMS Microbiol. Letts. 100:107–112
Tiwari R.P., Reeve W.G., Dilworth M.J., Glenn A.R. (1996a). An essential role for actA in acid tolerance of Rhizobium meliloti. Microbiology 142:601–610
Tiwari R.P., Reeve W.G. Dilworth M.J., Glenn A.R. (1996b). Acid tolerance in Rhizobium meliloti strain WSM419 involves a two-component sensor-regulatory system. Microbiology 142:1693–1704
Vincent J.M. (1970). A Manual for the Practical Study of Root Nodule Bacteria IBP Handbook No.15. Oxford, Blackwell Scientific
Vinuesa P., Neumann-Silkow F., Pacios-Bras C., Spaink H.P., Martinez-Romero E., Werner D. (2003). Genetic analysis of pH-regulated operon from Rhizobium tropici CIAT899 involved in acid tolerance and nodulation competitiveness. Mol. Plant-Microbe Interact. 16:159–168
Acknowledgements
This work was supported by the Royal Golden Jubilee Ph.D. Program of the Thailand Research Fund, Suranaree University of Technology, Nakhon Ratchasima, Thailand, and University of Hawaii, USA. We thank Jonathan Awaya for helpful suggestions and technical help throughout the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tittabutr, P., Payakapong, W., Teaumroong, N. et al. A histidine kinase sensor protein gene is necessary for induction of low pH tolerance in Sinorhizobium sp. strain BL3. Antonie Van Leeuwenhoek 89, 125–134 (2006). https://doi.org/10.1007/s10482-005-9015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-005-9015-0