Abstract
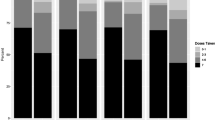
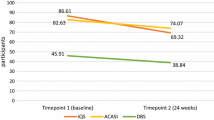
Understanding PrEP adherence is key in the formulation of HIV prevention strategies; however, measurement of adherence can be challenging. We compared multiple adherence measures in a two-year study of young Kenyan women at high risk of HIV acquisition. Among 289 participants, concordance between electronic adherence monitoring (EAM) and tenofovir diphosphate (TFV-DP) in dried blood spots ranged from 57 to 72% depending on selected thresholds. Using area under the receiver operating curve, discrimination of quantifiable TFV-DP was high at 0.85 with EAM and low at 0.49–0.54 for multiple self-reported measures. Correlation between EAM and self-reported measures was low (r < 0.11) although correlation within self-reported measures was moderate (r > 0.69). These findings indicate that both TFV-DP and EAM are useful PrEP adherence tools. Adherence would benefit from better availability of less expensive versions of both measurement tools. Additionally, further research on TFV-DP thresholds is needed to inform interpretation and use in understanding PrEP adherence in this population.
Resumen
Comprender la adherencia a la PrEP es importante en la formulación de estrategias de prevención del VIH; sin embargo, la medición de la adherencia puede ser difícil. Comparamos múltiples medidas de adherencia en un estudio de dos años de mujeres jóvenes kenianas con alto riesgo de contraer el VIH. Entre 289 participantes, la concordancia entre la monitorización electrónica de la adherencia (EAM) y el tenofovir difosfato (TFV-DP) en las manchas de sangre seca varió del 57% al 72% dependiendo de los umbrales seleccionados. Utilizando el área bajo la curva operativa del receptor, la discriminación de TFV-DP cuantificable fue alta en 0.85 con EAM y baja en 0.49–0.54 para múltiples medidas autoinformadas. La correlación entre la EAM y las medidas autoinformadas fue baja (r < 0,11), aunque la correlación entre de las medidas autoinformadas fue moderada (r > 0,69). Estos resultados indican que tanto TFV-DP como EAM son herramientas útiles de adherencia a la PrEP. La adherencia se beneficiaría de una mejor disponibilidad de versiones menos costosas de ambas herramientas de medición. Además, se necesita más investigación sobre los umbrales TFV-DP para informar la interpretación y el uso en la comprensión de la adherencia a la PrEP en esta población.

Similar content being viewed by others
Data availability
Data is available upon request.
Code availability
Statistical analysis code is available upon request.
References
UNAIDS.org. Global HIV & AIDS statistics - Fact sheet. 2020.
Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS. 2016;11:10–7.
CDC.gov. PrEP Eff. In; 2022.
Velloza J, Khoza N, Scorgie F, Chitukuta M, Mutero P, Mutiti K, et al. The influence of HIV-related stigma on PrEP disclosure and adherence among adolescent girls and young women in HPTN 082: a qualitative study. J Int AIDS Soc. 2020;23:e25463.
Celum CL, Delany-Moretlwe S, Baeten JM, van der Straten A, Hosek S, Bukusi EA, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc. 2019;22(Suppl 4):e25298.
Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure Prophylaxis for HIV infection among african women. N Engl J Med. 2012;367:411–22.
Celum CL, Bukusi EA, Bekker LG, Delany-Moretlwe S, Kidoguchi L, Omollo V, et al. PrEP use and HIV seroconversion rates in adolescent girls and young women from Kenya and South Africa: the POWER demonstration project. J Int AIDS Soc. 2022;25:e25962.
Celum C, Hosek S, Tsholwana M, Kassim S, Mukaka S, Dye BJ, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18:e1003670.
Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among african women. N Engl J Med. 2015;372:509–18.
Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis against HIV in Men and Women using Tenofovir Disoproxil Fumarate with or without Emtricitabine. J Infect Dis. 2016;214:55–64.
Chesney MA. The elusive gold standard. Future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):149–55.
Benitez AE, Musinguzi N, Bangsberg DR, Bwana MB, Muzoora C, Hunt PW, et al. Super learner analysis of real-time electronically monitored adherence to antiretroviral therapy under constrained optimization and comparison to non-differentiated care approaches for persons living with HIV in rural Uganda. J Int AIDS Soc. 2020;23:e25467.
Musinguzi N, Mocello RA, Boum Y, Hunt PW, Martin JN, Haberer JE, et al. Duration of viral suppression and risk of Rebound Viremia with First-Line Antiretroviral Therapy in Rural Uganda. AIDS Behav. 2017;21:1735–40.
Musinguzi N, Muganzi CD, Boum Y 2nd, Ronald A, Marzinke MA, Hendrix CW, et al. Comparison of subjective and objective adherence measures for preexposure prophylaxis against HIV infection among serodiscordant couples in East Africa. AIDS. 2016;30:1121–9.
Abaasa A, Hendrix C, Gandhi M, Anderson P, Kamali A, Kibengo F, et al. Utility of different adherence measures for PrEP: patterns and incremental value. AIDS Behav. 2018;22:1165–73.
Elbireer AM, Jackson JB, Sendagire H, Opio A, Bagenda D, Amukele TK. The good, the bad, and the unknown: quality of clinical laboratories in Kampala, Uganda. PLoS ONE. 2013;8:e64661.
Schroeder LF, Amukele T. Medical laboratories in sub-saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141:791–5.
Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–90.
Pyra M, Anderson P, Haberer JE, Heffron R, Celum C, Asiimwe S, et al. Tenofovir-Diphosphate as a marker of HIV Pre-exposure Prophylaxis Use among East African Men and Women. Front Pharmacol. 2019;10:401.
Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018,62.
Ibrahim ME, Castillo-Mancilla JR, Yager J, Brooks KM, Bushman L, Saba L, et al. Individualized adherence benchmarks for HIV Pre-Exposure Prophylaxis. AIDS Res Hum Retroviruses. 2021;37:421–8.
Mboup A, Béhanzin L, Guédou F, Giguère K, Geraldo N, Zannou DM, et al. Comparison of adherence measurement tools used in a pre-exposure prophylaxis demonstration study among female sex workers in Benin. Med (Baltim). 2020;99:e20063.
Haberer JE, Musinguzi N, Tsai AC, Boum Y 2nd, Bwana BM, Muzoora C, et al. Real-time electronic adherence monitoring plus follow-up improves adherence compared with standard electronic adherence monitoring. AIDS. 2017;31:169–71.
Haberer JE, Bukusi EA, Mugo NR, Pyra M, Kiptinness C, Oware K, et al. Effect of SMS reminders on PrEP adherence in young kenyan women (MPYA study): a randomised controlled trial. Lancet HIV. 2021;8:e130–7.
Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng JH, Ray ML, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56:390–401.
Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1907–14.
Wilson IB, Lee Y, Michaud J, Fowler FJ Jr, Rogers WH. Validation of a New Three-Item Self-Report measure for Medication Adherence. AIDS Behav. 2016;20:2700–8.
Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–9.
Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal. 2016;122:16–20.
Tape TG. Interpretation of diagnostic tests. Ann Intern Med. 2001;135:72.
Kenneth K, Mugwanya PLA. The Women TDF-FTC Benchmark Study. Kenya Medical Research Institute - Partners in Health Research and Development Thika, Kenya: National Institute of Allergy and Infectious Diseases (NIAID); 2022.
Blumenthal J, Pasipanodya EC, Jain S, Sun S, Ellorin E, Morris S, et al. Comparing Self-Report Pre-Exposure Prophylaxis adherence questions to pharmacologic measures of recent and cumulative pre-exposure Prophylaxis exposure. Front Pharmacol. 2019;10:721.
Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of measures of adherence to human immunodeficiency Virus Preexposure Prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis. 2018;66:213–9.
Musinguzi N, Muwonge T, Ngure K, Katabira E, Mugo N, Burns BFO, et al. Comparison of short messaging service self-reported adherence with other adherence measures in a demonstration project of HIV preexposure prophylaxis in Kenya and Uganda. AIDS. 2018;32:2237–45.
WHO. WHO recommends long-acting cabotegravir for HIV prevention. In; 2022.
UN.org. Gamechanger HIV injection rolls out in South Africa and Brazil. In; 2022.
Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring containing dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375:2121–32.
Acknowledgements
We would like to thank the study participants and the MPYA Study team below:
Investigators: Jessica E. Haberer, Jared M. Baeten, Elizabeth Bukusi, Nelly Mugo, Ruanne Barnabas, Harsha Thirumurthy, Ingrid Katz.
Kisumu study team: Kevin Kamolloh, Josephine Odoyo, Linda Aswani, Lawrence Juma, Elizabeth Koyo, Bernard Rono, Stanley Cheruiot, Vallery Ogello, Loice Okumu, Violet Kwach, Alfred Obiero, Stella Njuguna, Millicent Faith Akinyi, Lilian Adipo, Sylvia Akinyi.
Thika study team: Catherine Kiptiness, Nicholas Thuo, Stephen Gakuo Maina, Irene Njeru, Peter Mogere, Sarah Mbaire, Murugi Micheni, Lynda Oluoch, John Njoroge, Snaidah Ongachi, Jacinta Nyokabi.
Program manager: Lindsey Garrison.
Statisticians/analysts: Maria Pyra, Kathleen K. Thomas, Nicholas Musinguzi, Susie Valenzuela.
Clinical oversight: Susan Morrison.
Funding
This study was funded by the National Institute of Mental Health (R01MH109309); study medication was provided by Gilead (United States). Neither organization influenced the trial conduct, analysis, or presentation of the results.
Author information
Authors and Affiliations
Contributions
NM and JEH conceived and designed the analysis; NM performed the analysis; NM wrote the initial draft of the manuscript with significant input from JEH; KN, EAB, NRM, PLA, and JMB provided critical review of the manuscript.
Corresponding author
Ethics declarations
Ethics Statement
The study was approved by the Kenya Medical Research Institute (Nairobi, Kenya), MassGeneral Brigham (Boston, MA, USA), and the University of Washington (Seattle, WA, USA).
Consent to Participate
All participants gave written informed consent.
Consent for publication
Consent for publication was not requested since there was no relevant information collected as to necessitate this consent.
Conflict of interest
JEH reports personal fees from Merck, outside the submitted work. JMB is an employee of Gilead Sciences, outside the submitted work. All other authors declare no conflict of interest related to this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
EAB reports personal fees from Merck, outside the submitted work.
PLA reports personal fees from Gilead, Merck, ViiV and research support from Gilead paid to his institution all outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Musinguzi, N., Ngure, K., Bukusi, E.A. et al. Performance of Multiple Adherence Measures for pre-exposure Prophylaxis (PrEP) Among Young Women in Kenya. AIDS Behav 27, 3961–3969 (2023). https://doi.org/10.1007/s10461-023-04111-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-023-04111-2