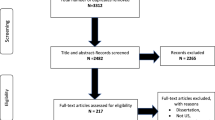
Abstract
HIV prevalence among cisgender female sex workers (FSW) and/or women who use drugs (WWUD) is substantially higher compared to similarly aged women. Consistent with PRISMA guidelines, we conducted the first systematic review on the pre-exposure prophylaxis (PrEP) continuum among FSW and/or WWUD, searching PubMed, Embase, CINAHL, PsycInfo, and Sociological Abstracts. Eligibility criteria included: reporting a PrEP related result among FSW and/or WWUD aged 18 + ; peer-reviewed; and published in English between 2012 and 2018. Our search identified 1365 studies; 26 met eligibility requirements, across the following groups: FSW (n = 14), WWUD (n = 9) and FSW-WWUD (n = 3). Studies report on at least one PrEP outcome: awareness (n = 12), acceptability (n = 16), uptake (n = 4), and adherence (n = 8). Specific barriers span individual and structural levels and include challenges to daily adherence, cost, and stigma. Combining health services and long-acting PrEP formulas may facilitate better PrEP uptake and adherence. The limited number of studies indicates a need for more research.
Resumen
La prevalencia del VIH es sustancialmente mayor entre trabajadoras sexuales cisgénero (FSW) y/o mujeres que usan drogas (WWUD) en comparación con mujeres de edad similar. Siguiendo PRISMA, realizamos la primera revisión sistemática sobre el continuo de la Profilaxis Preexposición (PrEP) entre estos grupos. Los criterios de elegibilidad fueron: informar un resultado de PrEP entre FSWs y/o WWUDs con mayores de edad; que haya pasado por revisión de pares; y publicados en inglés entre el 2012 y el 2018. Identificamos 1365 estudios; 26 cumplieron los criterios: FSW (n = 14), WWUD (n = 9) y FSW-WWUD (n = 3). Los estudios nos informaron sobre: conocimiento (n = 12), aceptabilidad (n = 16), uso, (n = 4) y adherencia (n = 8). Las barreras incluyen la adherencia, el costo, y el estigma. La combinación de los servicios de salud y de PrEP de acción-prolongada pueden facilitar mejor uso y adherencia a la PrEP. El número limitado de estudios indica la necesidad de más investigación.

Similar content being viewed by others
Abbreviations
- aOR:
-
Adjusted odds ratio
- ART:
-
Antiretroviral therapy
- CI:
-
Confidence interval
- FDA:
-
Food and Drug Administration
- FSW:
-
Female sex workers
- FSW-WWUD:
-
Female sex workers who are also women who use drugs
- IDU:
-
Injection drug users
- LMIC:
-
Lower and middle income countries
- MSM:
-
Men who have sex with men
- MPT:
-
Multipurpose technologies
- PrEP:
-
Pre-exposure prophylaxis
- PWID:
-
People who inject drugs
- PWUD:
-
Persons who use drugs
- RCT:
-
Randomized control trial
- SEP:
-
Syringe exchange program
- STIs:
-
Sexually transmitted infections
- TDF/FTC:
-
Tenofovir and emtricitabine medication
- WHO:
-
World Health Organization
- WWID:
-
Women who inject drugs
- WWUD:
-
Women who use drugs
References
Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–49.
Larney S, Mathers BM, Poteat T, Kamarulzaman A, Degenhardt L. Global epidemiology of HIV among women and girls who use or inject drugs: current knowledge and limitations of existing data. J Acquir Immune Defic Syndr. 2015;69(Suppl 2):100.
Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90.
World Health Organization. Policy brief: pre-exposure prophylaxis (PrEP): WHO expands recommendation on oral pre-exposure prophylaxis of HIV infection (PrEP). Geneva: World Health Organization; 2015.
Cleland CM, Des Jarlais DC, Perlis TE, Stimson G, Poznyak V. HIV risk behaviors among female IDUs in developing and transitional countries. BMC Public Health. 2007;7(1):271.
Roberts A, Mathers B, Degenhardt L. Women who inject drugs: a review of their risks, experiences and needs. A report prepared on behalf of the Reference Group to the United Nations on HIV and injecting drug use. Sydney: University of New South Wales; 2010.
Astemborski J, Vlahov D, Warren D, Solomon L, Nelson KE. The trading of sex for drugs or money and HIV seropositivity among female intravenous drug users. Am J Public Health. 1994;84(3):382–7.
Baral S, Todd CS, Aumakhan B, Lloyd J, Delegchoimbol A, Sabin K. HIV among female sex workers in the Central Asian Republics, Afghanistan, and Mongolia: contexts and convergence with drug use. Drug Alcohol Depend. 2013;132:S13–6.
Loza O, Patterson TL, Rusch M, Martínez GA, Lozada R, Staines-Orozco H, et al. Drug-related behaviors independently associated with syphilis infection among female sex workers in two Mexico–US border cities. Addiction. 2010;105(8):1448–56.
Patterson TL, Semple SJ, Staines H, Lozada R, Orozovich P, Bucardo J, et al. Prevalence and correlates of HIV infection among female sex workers in 2 Mexico—US border cities. J Infect Dis. 2008;197(5):728–32.
Sanders T. A continuum of risk? The management of health, physical and emotional risks by female sex workers. Sociol Health Illness. 2004;26(5):557–74.
Abad N, Baack BN, O’Leary A, Mizuno Y, Herbst JH, Lyles CM. A systematic review of HIV and STI behavior change interventions for female sex workers in the United States. AIDS Behav. 2015;19(9):1701–19.
Surratt HL, Kurtz SP, Chen M, Mooss A. HIV risk among female sex workers in Miami: the impact of violent victimization and untreated mental illness. AIDS Care. 2012;24(5):553–61.
Buttram ME, Surratt HL, Kurtz SP. Resilience and syndemic risk factors among African-American female sex workers. Psychol Health Med. 2014;19(4):442–52.
Shannon K, Kerr T, Allinott S, Chettiar J, Shoveller J, Tyndall MW. Social and structural violence and power relations in mitigating HIV risk of drug-using women in survival sex work. Soc Sci Med. 2008;66(4):911–21.
Footer KH, Silberzahn BE, Tormohlen KN, Sherman SG. Policing practices as a structural determinant for HIV among sex workers: a systematic review of empirical findings. J Int AIDS Soc. 2016;19:20883.
El-Bassel N, Shaw SA, Dasgupta A, Strathdee SA. Drug use as a driver of HIV risks: re-emerging and emerging issues. Curr Opin HIV AIDS. 2014;9(2):150.
Des Jarlais DC, Feelemyer JP, Modi SN, Arasteh K, Hagan H. Are females who inject drugs at higher risk for HIV infection than males who inject drugs: an international systematic review of high seroprevalence areas. Drug Alcohol Depend. 2012;124(1):95–107.
U.S. Department of Health & Human Services, U.S. Food and Drug Administration. Truvada for PrEP fact sheet: ensuring safe and proper use. 2012. https://www.fda.gov/media/83586/download. Accessed 8 Oct 2018.
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34.
Van Der Straten A, Brown ER, Marrazzo JM, Chirenje MZ, Liu K, Gomez K, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc. 2016;19(1):20642.
Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22.
Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71.
Duff P, Goldenberg S, Deering K, Montaner J, Nguyen P, Dobrer S, et al. Barriers to viral suppression among female sex workers: role of structural and intimate partner dynamics. J Acquir Immune Defic Syndr. 2016;73(1):83.
Yi S, Tuot S, Mwai GW, Ngin C, Chhim K, Pal K, et al. Awareness and willingness to use HIV pre-exposure prophylaxis among men who have sex with men in low-and middle-income countries: a systematic review and meta-analysis. J Int AIDS Soc. 2017;20(1):21580.
Eakle R, Bourne A, Mbogua J, Mutanha N, Rees H. Exploring acceptability of oral PrEP prior to implementation among female sex workers in South Africa. J Int AIDS Soc. 2018;21(2):e25081.
UNAIDS. Pre-exposure prophylaxis (PrEP). http://www.unaids.org/en/resources/presscentre/featurestories/2016/october/20161031_PrEP. Accessed 8 Oct 2018.
Kelley CF, Kahle E, Siegler A, Sanchez T, Del Rio C, Sullivan PS, et al. Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis. 2015;61(10):1590–7.
Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, Mayer KH, Mimiaga M, Patel R, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS. 2017;31(5):731.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Peacock R. Storylines of research in diffusion of innovation: a meta-narrative approach to systematic review. Soc Sci Med. 2005;61(2):417–30.
Walters SM, Rivera AV, Starbuck L, Reilly KH, Boldon N, Anderson BJ, et al. Differences in awareness of pre-exposure prophylaxis and post-exposure prophylaxis among groups at-risk for HIV in New York State: New York City and Long Island, NY, 2011–2013. J Acquir Immune Defic Syndr. 2017;75(Suppl 3):S383–91.
Gnatienko N, Wagman JA, Cheng DM, Bazzi AR, Raj A, Blokhina E, et al. Serodiscordant partnerships and opportunities for pre-exposure prophylaxis among partners of women and men living with HIV in St Petersburg, Russia. PLoS ONE. 2018;13(11):e0207402.
Roth A, Tran N, Piecara B, Welles S, Shinefeld J, Brady K. Factors associated with awareness of pre-exposure prophylaxis for HIV among persons who inject drugs in Philadelphia: National HIV Behavioral Surveillance. AIDS Behav. 2015;2018:1–8.
Peng B, Yang X, Zhang Y, Dai J, Liang H, Zou Y, et al. Willingness to use pre-exposure prophylaxis for HIV prevention among female sex workers: a cross-sectional study in China. HIV/AIDS Res Palliat Care. 2012;4:149–58.
Ye L, Wei S, Zou Y, Yang X, Abdullah AS, Zhong X, et al. HIV pre-exposure prophylaxis interest among female sex workers in Guangxi, China. PLoS ONE. 2014;9(1):e86200.
Walters SM, Reilly KH, Neaigus A, Braunstein S. Awareness of pre-exposure prophylaxis (PrEP) among women who inject drugs in NYC: the importance of networks and syringe exchange programs for HIV prevention. Harm Reduct J. 2017;14(1):40.
Peitzmeier SM, Tomko C, Wingo E, Sawyer A, Sherman SG, Glass N, et al. Acceptability of microbicidal vaginal rings and oral pre-exposure prophylaxis for HIV prevention among female sex workers in a high-prevalence US city. AIDS Care. 2017;29(11):1453–7.
Jackson T, Huang A, Chen H, Gao X, Zhang Y, Zhong X. Predictors of willingness to use HIV pre-exposure prophylaxis among female sex workers in Southwest China. AIDS Care. 2013;25(5):601–5.
Ortblad KF, Chanda MM, Musoke DK, Ngabirano T, Mwale M, Nakitende A, et al. Acceptability of HIV self-testing to support pre-exposure prophylaxis among female sex workers in Uganda and Zambia: results from two randomized controlled trials. BMC Infect Dis. 2018;18(1):503.
Mack N, Evens EM, Tolley EE, Brelsford K, Mackenzie C, Milford C, et al. The importance of choice in the rollout of ARV-based prevention to user groups in Kenya and South Africa: a qualitative study. J Int AIDS Soc. 2014;17(3 Suppl 2):19157.
Bazzi AR, Yotebieng KA, Agot K, Rota G, Syvertsen JL. Perspectives on biomedical HIV prevention options among women who inject drugs in Kenya. AIDS Care. 2018;30(3):343–6.
Restar AJ, Tocco JU, Mantell JE, Lafort Y, Gichangi P, Masvawure TB, et al. Perspectives on HIV pre- and post-exposure prophylaxes (PrEP and PEP) Among female and male sex workers in Mombasa, Kenya: implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29(2):141–53.
Reza-Paul S, Lazarus L, Doshi M, Hafeez Ur Rahman S, Ramaiah M, Maiya R, et al. Prioritizing risk in preparation for a demonstration project: a mixed methods feasibility study of oral pre-exposure prophylaxis (PREP) among female sex workers in South India. PLoS ONE. 2016;11(11):0166889.
Garfinkel DB, Alexander KA, McDonald-Mosley R, Willie TC, Decker MR. Predictors of HIV-related risk perception and PrEP acceptability among young adult female family planning patients. AIDS Care. 2017;29(6):751–8.
Stein M, Thurmond P, Bailey G. Willingness to use HIV pre-exposure prophylaxis among opiate users. AIDS Behav. 2014;18(9):1694–700.
Shrestha R, Karki P, Altice FL, Huedo-Medina TB, Meyer JP, Madden L, et al. Correlates of willingness to initiate pre-exposure prophylaxis and anticipation of practicing safer drug- and sex-related behaviors among high-risk drug users on methadone treatment. Drug Alcohol Depend. 2017;173:107–16.
Escudero DJ, Kerr T, Wood E, Nguyen P, Lurie MN, Sued O, et al. Acceptability of HIV pre-exposure prophylaxis (PREP) among people who inject drugs (PWID) in a Canadian setting. AIDS Behav. 2015;19(5):752–7.
Roth AM, Aumaier BL, Felsher MA, Welles SL, Martinez-Donate AP, Chavis M, et al. An exploration of factors impacting preexposure prophylaxis eligibility and access among syringe exchange users. Sex Transm Dis. 2018;45(4):217–21.
Pines H, Strathdee S, Hendrix C, Bristow C, Harvey-Vera A, Magis-Rodríguez C, et al. Oral and vaginal HIV pre-exposure prophylaxis product attribute preferences among female sex workers in the Mexico-US border region. Int J STD AIDS. 2019;30(1):45–55.
Wanyenze RK, Musinguzi G, Kiguli J, Nuwaha F, Mujisha G, Musinguzi J, et al. “When they know that you are a sex worker, you will be the last person to be treated”: perceptions and experiences of female sex workers in accessing HIV services in Uganda. BMC Int Health Hum Rights Q. 2017;17(1):11.
Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med. 2017;14(11):e1002444.
Mboup A, Béhanzin L, Guédou FA, Geraldo N, Goma-Matsétsé E, Giguère K, et al. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc. 2018;21(11):e25208.
Cowan FM, Davey C, Fearon E, Mushati P, Dirawo J, Chabata S, et al. Targeted combination prevention to support female sex workers in Zimbabwe accessing and adhering to antiretrovirals for treatment and prevention of HIV (SAPPH-IRe): a cluster-randomised trial. Lancet HIV. 2018;5(8):e417–26.
Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, et al. Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. Lancet HIV. 2017;4(2):e59–66.
Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. AIDS (London, England). 2015;29(7):819–24.
Prüss-Ustün A, Wolf J, Driscoll T, Degenhardt L, Neira M, Calleja JMG. HIV due to female sex work: regional and global estimates. PLoS ONE. 2013;8(5):e63476.
UNAIDS Geneva. Unaids guidance note on HIV and sex work. 2009. https://www.unaids.org/sites/default/files/sub_landing/files/JC2306_UNAIDS-guidance-note-HIV-sex-work_en.pdf. Accessed 8 Oct 2018.
Li J, Berg CJ, Kramer MR, Haardörfer R, Zlotorzynska M, Sanchez TH. An integrated examination of county-and individual-level factors in relation to HIV pre-exposure prophylaxis awareness, willingness to use, and uptake among men who have sex with men in the US. AIDS Behav. 2019;23(7):1721–36.
Bekker L-G, Johnson L, Cowan F, Overs C, Besada D, Hillier S, et al. Combination HIV prevention for female sex workers: what is the evidence? Lancet. 2015;385(9962):72–87.
Friend DR, Clark JT, Kiser PF, Clark MR. Multipurpose prevention technologies: products in development. J Antivir Res. 2013;100:S39–47.
Pinto RM, Berringer KR, Melendez R, Mmeje O. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22(11):3681–91.
Bailey JL, Molino ST, Vega AD, Badowski M. A review of HIV pre-exposure prophylaxis: the female perspective. Infect Dis Ther. 2017;6(3):363–82.
Benítez-Gutiérrez L, Soriano V, Requena S, Arias A, Barreiro P, de Mendoza C. Treatment and prevention of HIV infection with long-acting antiretrovirals. Expert Rev Clin Pharmacol. 2018;11(5):507–17.
Romano JW, Van Damme L, Hillier S. The future of multipurpose prevention technology product strategies: understanding the market in parallel with product development. BJOG. 2014;121:15–8.
Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4.
Hoornenborg E, Krakower DS, Prins M, Mayer KH. Pre-exposure prophylaxis for MSM and transgender persons in early adopting countries. AIDS. 2017;31(16):2179–91.
Karki P, Shrestha R, Huedo-Medina TB, Copenhaver M. The impact of methadone maintenance treatment on HIV risk behaviors among high-risk injection drug users: a systematic review. Evid Based Med Public Health. 2016;2:e1229.
Wong K-h, Lee S-s, Lim W-l, Low H-k. Adherence to methadone is associated with a lower level of HIV-related risk behaviors in drug users. J Subst Abuse Treat. 2003;24(3):233–9.
Shrestha R, Copenhaver M. Exploring the use of pre-exposure prophylaxis (PrEP) for HIV prevention among high-risk people who use drugs in treatment. Front Public Health. 2018;6:195.
Shrestha R, Altice F, Karki P, Copenhaver M. Developing an integrated, brief biobehavioral HIV prevention intervention for high-risk drug users in treatment: the process and outcome of formative research. Front Immunol. 2017;8:561.
Goldenberg SM, Montaner J, Duff P, Nguyen P, Dobrer S, Guillemi S, et al. Structural barriers to antiretroviral therapy among sex workers living with HIV: findings of a longitudinal study in Vancouver, Canada. AIDS Behav. 2016;20(5):977–86.
Cornelius T, Jones M, Merly C, Welles B, Kalichman MO, Kalichman SC. Impact of food, housing, and transportation insecurity on ART adherence: a hierarchical resources approach. AIDS Care. 2017;29(4):449–57.
Acknowledgements
We thank the authors of the included studies. We also thank Kenneth Morales, MPH for his assistance in the title abstract review process. This work was supported by the NIH/NIDA (R34DA045619-02), the Drug Dependence Epidemiology Training Program, NIH/NIDA (T32DA007292), the Johns Hopkins University Hopkins Population Center (R24HD042854) and the Johns Hopkins Center for AIDS Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Glick, J.L., Russo, R., Jivapong, B. et al. The PrEP Care Continuum Among Cisgender Women Who Sell Sex and/or Use Drugs Globally: A Systematic Review. AIDS Behav 24, 1312–1333 (2020). https://doi.org/10.1007/s10461-019-02733-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-019-02733-z