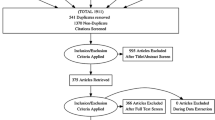
Abstract
This randomized behavioral trial examined whether youth living with HIV (YLH) receiving cell-phone support with study funded phone plans, demonstrated improved adherence and viral control during the 24 week intervention and 24 weeks post-intervention compared to controls. Monday through Friday phone calls confirmed medications were taken, provided problem-solving support, and referred to services to address adherence barriers. Of 37 participants (ages 15–24), 62 % were male and 70 % were African American. Self-reported adherence was significantly higher in the intervention group compared to the control at 24 and 48 weeks for the past month (P = 0.007) and log 10 HIV VL was significantly lower at both 24 weeks (2.82 versus 4.52 P = 0.002) and 48 weeks (3.23 versus 4.23 P = 0.043). Adherence and viral load showed medium to large effect sizes across the 48 week study. This is the first study to demonstrate sustained clinically significant reductions in HIV VL using youth friendly technology.
Resumen
Este ensayo de comportamiento aleatorio examinó si los jóvenes que viven con el VIH (YLH) reciben apoyo por celular con el estudio financiado de teléfono, se demostró un mejor cumplimiento y control viral durante la intervención de 24 semanas y 24 semanas después de la intervención en comparación con los controles. De Lunes a Viernes las llamadas telefónicas confirmaron los medicamentos, apoyaron la resolución de problemas, y se refirió a los servicios para hacer frente a las barreras de adherencia. De los 37 participantes (siglos 15–24), el 62 % eran hombres y el 70 % eran afroamericanos. Adherencia auto-reportada fue significativamente mayor en el grupo de intervención en comparación con el control a las 24 y 48 semanas del mes pasado (P = 0.007) y el log 10 VL VIH fue significativamente menor en ambos 24 semanas (2.82 versus 4.52 P = 0.002) y 48 semanas (3.23 versus 4.23 P = 0.043). La adhesión y la carga viral mostraron medianas y grandes tamaños del efecto en todo el estudio de 48 semanas. Este es el primer estudio que demuestra descensos importantes de la VL VIH utilizando tecnología amigable para la juventud.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
Belzer ME, OJ. Adherence in adolescents: a review of the literature. Adolescent medicine: state of the art reviews evaluation and management of adolescent issues. [Review article]. 2008;19(1):99–118.
Policy TWHOoNA. National HIV/AIDS strategy for the United States. Washington DC 2010.
Comulada WS, Swendeman DT, Rotheram-Borus MJ, Mattes KM, Weiss RE. Use of HAART among young people living with HIV. Am J Health Behav. 2003;27(4):389–400.
Macdonell KE, Naar-King S, Murphy DA, Parsons JT, Harper GW. Predictors of medication adherence in high risk youth of color living with HIV. J Pediatr Psychol. 2010;35(6):593–601.
Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR, et al. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157(3):249–55.
MacDonell K, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS behav. 2013;17(1):86–93.
Gaur AH, Belzer M, Britto P, Garvie PA, Hu CC, Graham B, et al. Directly observed therapy (DOT) for nonadherent HIV-infected youth: lessons learned challenges ahead. Aids Res Hum Retrov. 2010;26(9):947–53.
Garvie PA, Flynn PM, Belzer M, Britto P, Hu C, Graham B, et al. Psychological factors, beliefs about medication, and adherence of youth with human immunodeficiency virus in a multisite directly observed therapy pilot study. J Adolesc Health. 2011;48(6):637–40.
Naar-King S, Parsons JT, Murphy DA, Chen XG, Harris DR, Belzer ME. Improving health outcomes for youth living with the human immunodeficiency virus a multisite randomized trial of a motivational intervention targeting multiple risk behaviors. Arch Pediatr Adolesc Med. 2009;163(12):1092–8.
Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143–67.
Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165–73.
Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32(1):56–69.
Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Internet Res. 2012;14(2):e51. doi:10.2196/jmir.2015.
Reynolds NR, Testa MA, Su M, Chesney MA, Neidig JL, Frank I, et al. Telephone support to improve antiretroviral medication adherence—a multisite, randomized controlled trial. Jaids-J Acquir Immuno Defic. 2008;47(1):62–8.
Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45.
Pop-Elrches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in resource-limited setting: a randomized controlled trial of text message reminders. AIDS Behav. 2011;25(6):825–34.
Puccio JA, Belzer M, Olson J, Martinez M, Salata C, Tucker D, et al. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. Aids Patient Care and STDS. 2006;20(6):438–44.
Mallinson RK, Rajabiun S, Coleman S. The provider role in client engagement in HIV care. Aids Patient Care STDS. 2007;21:S77–84.
Murphy DA, Marelich WD, Hoffman D, Steers WN. Predictors of antiretroviral adherence. Aids Care: Psychol Socio-Med Asp Aids/Hiv. 2004;16(4):471–84.
Gardenier D, Andrews CM, Thomas DC, Bookhardt-Murray LJ, Fitzpatrick JJ. Social support and adherence: differences among clients in an AIDS day health care program. J Assoc Nurses AIDS Care. 2010;21(1):75–85.
Murphy DA, Marelich WD, Hoffman D, Steers WN. Predictors of antiretroviral adherence. AIDS Care. 2004;16(4):471–84.
Singh N, Berman SM, Swindells S, Justis JC, Mohr JA, Squier C, et al. Adherence of human immunodeficiency virus-infected patients to antiretroviral therapy. Clin Infect Dis. 1999;29(4):824–30.
Mallinson RK, Rajabiun S, Coleman S. The provider role in client engagement in HIV care. AIDS Patient Care STDS. 2007;21(Suppl 1):S77–84.
Diggle P, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press; 1994.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: L. Erlbaum Associates; 1988.
Acknowledgments
This work was supported by The Adolescent Trials Network for HIV/AIDS Interventions (ATN; 5U01-HD 40533 and 5 UO1 HD 40474) from the National Institutes of Health through the National Institute of Child Health and Human Development (B. Kapogiannis, S. Lee), with supplemental funding from the National Institutes of Drug Abuse (S. Kahana) and Mental Health (P. Brouwers, S. Allison). The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at the University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz, B. Driver). We acknowledge the contribution of the investigators and staff at the following ATN 078 sites that participated in this study: Children’s Hospital of Los Angeles (Marvin Belzer, M.D., Julie McAvoy-Banerjea, MPH, Michelle Bradford, B.A.); Children’s National Medical Center (Lawrence D’Angelo, M.D., Connie Trexler, RN, Amanda Terry, BS); University of California, San Francisco (Barbara Moscicki, M.D., Lisa Irish, B.S.N., Nigel R. Reyes, BFA); Tulane Medical Center (Sue Ellen Abdalian, M.D., Brenda H Andrews, MSN, Heather J. Ray, BS); Children’s Diagnostic & Treatment Center (Ana Puga, M.D., Amy Inman, BS, James S. Blood, MSW); We sincerely thank the ATN 078 Protocol Team Members (Steven Asch, M.D., Aditya Gaur, M.D., Sue Ellen Abdalian, M.D., Esmine Leonard, BSN, Trina Jeanjacques, BA, Catherine Forbes, PhD), the ATN Community Advisory Board, and the youth who participated in the study. An oral presentation of portions of this study was made at the bi-annual Adolescent Medicine Trials Network for HIV/AIDS Intervention Meeting in Bethesda, MD, on October 2, 2012. The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of NIDA or any of the sponsoring organizations, agencies, or the US government.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Belzer, M.E., Naar-King, S., Olson, J. et al. The Use of Cell Phone Support for Non-adherent HIV-Infected Youth and Young Adults: An Initial Randomized and Controlled Intervention Trial. AIDS Behav 18, 686–696 (2014). https://doi.org/10.1007/s10461-013-0661-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-013-0661-3
Keywords
Profiles
- Moussa Sarr View author profile