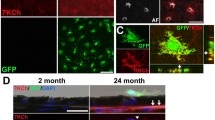
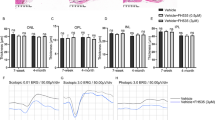
Abstract
Oxidized cholesterols and lipids accumulate in Bruch’s membrane in age-related macular degeneration (AMD). It remains unknown what causal relationship exists between these substances and AMD pathophysiology. We addressed the hypothesis that a prevalent form, 7-ketocholesterol (7KC), promotes choroidal endothelial cell (CEC) migration and macular neovascularization in AMD. Compared to control, 7KC injection caused 40% larger lectin-stained lesions, but 70% larger lesions measured by optical coherence tomography one week after laser-injury. At two weeks, 7KC-injected eyes had 86% larger alpha smooth muscle actin (αSMA)-labeled lesions and more collagen-labeling than control. There was no difference in cell death. 7KC-treated RPE/choroids had increased αSMA but decreased VE-cadherin. Compared to control-treated CECs, 7KC unexpectedly reduced endothelial VE-cadherin, CD31 and VEGFR2 and increased αSMA, fibroblast activation protein (FAP) and transforming growth factor beta (TGFβ). Inhibition of TGFβ receptor-mediated signaling by SB431542 abrogated 7KC-induced loss of endothelial and increase in mesenchymal proteins in association with decreased transcription factor, SMAD3. Knockdown of SMAD3 partially inhibited 7KC-mediated loss of endothelial proteins and increase in αSMA and FAP. Compared to control, 7KC-treatment of CECs increased Rac1GTP and migration, and both were inhibited by the Rac1 inhibitor; however, CECs treated with 7KC had reduced tube formation. These findings suggest that 7KC, which increases in AMD and with age, induces mesenchymal transition in CECs making them invasive and migratory, and causing fibrosis in macular neovascularization. Further studies to interfere with this process may reduce fibrosis and improve responsiveness to anti-VEGF treatment in non-responsive macular neovascularization in AMD.









Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Blasiak J et al (2014) Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res Int 2014:768026
Wang H, Hartnett ME (2016) Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis 22:189–202
Evans RN et al (2020) Associations of Variation in Retinal Thickness With Visual Acuity and Anatomic Outcomes in Eyes With Neovascular Age-Related Macular Degeneration Lesions Treated With Anti-Vascular Endothelial Growth Factor Agents. JAMA Ophthalmol
Bhisitkul RB et al (2015) Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol 159(5): 915-24 e2.
Cheung CMG et al (2019) The evolution of fibrosis and atrophy and their relationship with visual outcomes in asian persons with neovascular age-related macular degeneration. Ophthalmol Retina 3(12):1045–1055
Fritsche LG et al (2014) Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet 15:151–71
Seddon JM et al (2007) Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA: J Am Med Assoc 297(16):1793–1800
Domalpally A et al (2019) Prevalence, risk, and genetic association of reticular pseudodrusen in age-related macular degeneration: age-related eye disease study 2 report 21. Ophthalmology 126(12):1659–1666
Merle BM et al (2016) Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr 103(4):1135–44
Fernandez-Godino R (2018) Alterations in extracellular matrix/bruch’s membrane can cause the activation of the alternative complement pathway via tick-over. Adv Exp Med Biol 1074:29–35
Thulliez M et al (2019) Correlations between choriocapillaris flow deficits around geographic atrophy and enlargement rates based on swept-source OCT imaging. Ophthalmol Retina 3(6):478–488
Lamin A et al (2019) Changes in volume of various retinal layers over time in early and intermediate age-related macular degeneration. Eye (Lond) 33(3):428–434
Freund KB, Zweifel SA, Engelbert M (2010) Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 30(9):1333–49
Peterson LJ et al (2007) Heterotypic RPE-choroidal endothelial cell contact increases choroidal endothelial cell transmigration via PI 3-kinase and Rac1. Exp Eye Res 84(4):737–744
Wang H et al (2015) Rap1 GTPase inhibits tumor necrosis factor-alpha-induced choroidal endothelial migration via NADPH oxidase- and NF-kappaB-dependent activation of Rac1. Am J Pathol 185(12):3316–25
Monaghan-Benson E et al (2010) The Role of vascular endothelial growth factor-induced activation of NADPH oxidase in choroidal endothelial cells and choroidal neovascularization. Am J Pathol 177(4):2091–2102
Monaghan-Benson E, Burridge K (2009) The regulation of VEGF-induced microvascular permeability requires Rac and ROS. J Biol Chem M109
Wang H et al (2011) Upregulation of CCR3 by age-related stresses promotes choroidal endothelial cell migration via VEGF-dependent and -independent signaling. Investigat Ophthalmol Visual Sci 52(11):8271–8277
Wang H et al (2016) Retinal inhibition of CCR3 induces retinal cell death in a murine model of choroidal neovascularization. PLoS One 11(6):e0157748
Anderson A et al (2020) 7-Ketocholesterol in disease and aging. Redox Biol 29:101380
Rodriguez IR, Larrayoz IM (2010) Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J Lipid Res 51(10):2847–62
Rodriguez IR et al (2014) 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp Eye Res 128:151–5
Crabb JW et al (2020) Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proceed Natl Acad Sci 222551899.
Fliesler SJ (2015) Cholesterol homeostasis in the retina: seeing is believing. J Lipid Res 56(1):1–4
Curcio CA et al (2001) Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci 42(1):265–74
Curcio CA et al (2005) Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res 81(6):731–741
Vejux A et al (2020) 7-Ketocholesterol and 7β-hydroxycholesterol: In vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity. Biochem Pharmacol 173:113648
Dugas B et al (2010) Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur J Nutr 49(7):435–46
Chakravarthy U et al (2020) Progression from early/intermediate to advanced forms of age-related macular degeneration in a large UK cohort: rates and risk factors. Ophthalmol Retina 4(7):662–672
Wang H et al (2020) IQGAP1 causes choroidal neovascularization by sustaining VEGFR2-mediated Rac1 activation. Angiogenesis
Becker S et al (2018) Targeted Knockdown of Overexpressed VEGFA or VEGF164 in Muller cells maintains retinal function by triggering different signaling mechanisms. Sci Rep 8(1):2003
Li W et al (2011) Lipid accumulation and lysosomal pathways contribute to dysfunction and apoptosis of human endothelial cells caused by 7-oxysterols. Biochem Biophys Res Commun 409(4):711–6
Inagaki S et al (2020) Anti-vascular endothelial growth factor antibody limits vascular leakage and decreases subretinal fibrosis in a cynomolgus monkey choroidal neovascularization model. Curr Neurovasc Res
Shu DY, Butcher E, Saint-Geniez M (2020) EMT and EndMT: emerging roles in age-related macular degeneration. Int J Mol Sci 21(12)
Alyaseer AAA, de Lima MHS, Braga TT (2020) The role of NLRP3 inflammasome activation in the epithelial to mesenchymal transition process during the fibrosis. Front Immunol 11:883
Radeke MJ et al (2015) Restoration of mesenchymal retinal pigmented epithelial cells by TGFbeta pathway inhibitors: implications for age-related macular degeneration. Genome Med 7(1):58
Bischoff J (2019) Endothelial-to-mesenchymal transition. Circ Res 124(8):1163–1165
Schmierer B, Hill CS (2007) TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8(12):970–82
Syed V (2016) TGF-β signaling in cancer. J Cell Biochem 117(6):1279–87
Gurzu S et al (2019) Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. Biomed Res Int 2019:2962580
Rossato FA et al. (2020) Fibrotic changes and endothelial-to-mesenchymal transition promoted by VEGFR2 antagonism alter the therapeutic effects of VEGFA pathway blockage in a mouse model of choroidal neovascularization. Cells 9(9).
Piera-Velazquez S, Jimenez SA (2019) Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev 99(2):1281–1324
Souilhol C et al (2018) Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res 114(4):565–577
Nowak-Sliwinska P et al (2018) Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21(3):425–532
Wang H et al (2011) The role of RPE cell-associated VEGF189 in choroidal endothelial cell transmigration across the RPE. Investigat Ophthalmol Visual Sci 52(1):570–578
Acknowledgments
This work was supported by the National Institutes of Health EY014800 and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah; and the National Institutes of Health R01EY015130 and R01EY017011 to M.E.H.
Author information
Authors and Affiliations
Contributions
HW developed the hypothesis, designed and performed the experiments and wrote the manuscript; AR and EK performed experiments, maintained the animals and reviewed the manuscript; MEH developed the hypothesis, designed the experiments, wrote the manuscript, provided funding support and oversaw lab proceedings.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared there were no conflicts of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Ramshekar, A., Kunz, E. et al. 7-ketocholesterol induces endothelial-mesenchymal transition and promotes fibrosis: implications in neovascular age-related macular degeneration and treatment. Angiogenesis 24, 583–595 (2021). https://doi.org/10.1007/s10456-021-09770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-021-09770-0