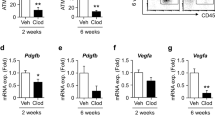
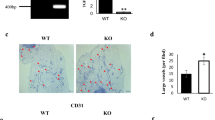
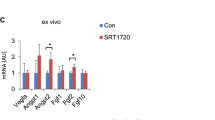
Abstract
Platelet-derived growth factor-B (PDGF-B) is a main factor to promote adipose tissue angiogenesis, which is responsible for the tissue expansion in obesity. In this process, PDGF-B induces the dissociation of pericytes from blood vessels; however, its regulatory mechanism remains unclear. In the present study, we found that stromal cell-derived factor 1 (SDF1) plays an essential role in this regulatory mechanism. SDF1 mRNA was increased in epididymal white adipose tissue (eWAT) of obese mice. Ex vivo pharmacological analyses using cultured adipose tissue demonstrated that physiological concentrations (1–100 pg/mL) of SDF1 inhibited the PDGF-B-induced pericyte dissociation from vessels via two cognate SDF1 receptors, CXCR4 and CXCR7. In contrast, higher concentrations (> 1 ng/mL) of SDF1 alone caused the dissociation of pericytes via CXCR4, and this effect disappeared in the cultured tissues from PDGF receptor β (PDGFRβ) knockout mice. To investigate the role of SDF1 in angiogenesis in vivo, the effects of anagliptin, an inhibitor of dipeptidyl peptidase 4 (DPP4) that degrades SDF1, were examined in mice fed a high-fat diet. Anagliptin increased the SDF1 levels in the serum and eWAT. These changes were associated with a reduction of pericyte dissociation and fat accumulation in eWAT. AMD3100, a CXCR4 antagonist, cancelled these anagliptin effects. In flow-cytometry analysis, anagliptin increased and decreased the PDGF-B expression in endothelial cells and macrophages, respectively, whereas anagliptin reduced the PDGFRβ expression in pericytes of eWAT. These results suggest that SDF1 negatively regulates the adipose tissue angiogenesis in obesity by altering the reactivity of pericytes to PDGF-B.









Similar content being viewed by others
References
Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ (2002) Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99:10730–10735. https://doi.org/10.1073/pnas.162349799
Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W (2004) Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10:625–632. https://doi.org/10.1038/nm1048
Cao Y (2010) Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 9:107–115. https://doi.org/10.1038/nrd3055
Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A (2013) Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. https://doi.org/10.1016/j.cmet.2012.12.010
di Somma M, Vliora M, Grillo E, Castro B, Dakou E, Schaafsma W, Vanparijs J, Corsini M, Ravelli C, Sakellariou E, Mitola S (2019) Role of VEGFs in metabolic disorders. Angiogenesis. https://doi.org/10.1007/s10456-019-09700-1
Onogi Y, Wada T, Kamiya C, Inata K, Matsuzawa T, Inaba Y, Kimura K, Inoue H, Yamamoto S, Ishii Y, Koya D, Tsuneki H, Sasahara M, Sasaoka T (2017) PDGFRβ regulates adipose tissue expansion and glucose metabolism via vascular remodeling in diet-induced obesity. Diabetes 66:1008–1021. https://doi.org/10.2337/db16-0881
Onogi Y, Wada T, Okekawa A, Matsuzawa T, Watanabe E, Ikeda K, Nakano M, Kitada M, Koya D, Tsuneki H, Sasaoka T (2020) Pro-inflammatory macrophages coupled with glycolysis remodel adipose vasculature by producing platelet-derived growth factor-B in obesity. Sci Rep 10:670. https://doi.org/10.1038/s41598-019-57368-w
Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124:175–189. https://doi.org/10.1016/j.cell.2005.10.036
Petit I, Jin D, Rafii S (2007) The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 28:299–307. https://doi.org/10.1016/j.it.2007.05.007
Singh AK, Arya RK, Trivedi AK, Sanyal S, Baral R, Dormond O, Briscoe DM, Datta D (2013) Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev 24:41–49. https://doi.org/10.1016/j.cytogfr.2012.08.007
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864. https://doi.org/10.1038/nm1075
Yamada K, Maishi N, Akiyama K, Towfik Alam M, Ohga N, Kawamoto T, Shindoh M, Takahashi N, Kamiyama T, Hida Y, Taketomi A, Hida K (2015) CXCL12-CXCR7 axis is important for tumor endothelial cell angiogenic property. Int J Cancer 137:2825–2836. https://doi.org/10.1002/ijc.29655
Sánchez-Martín L, Sánchez-Mateos P, Cabañas C (2013) CXCR7 impact on CXCL12 biology and disease. Trends Mol Med 19:12–22. https://doi.org/10.1016/j.molmed.2012.10.004
Zhong J, Rajagopalan S (2015) Dipeptidyl peptidase-4 regulation of SDF-1/CXCR4 Axis: implications for cardiovascular disease. Front Immunol 6:477. https://doi.org/10.3389/fimmu.2015.00477
Mulvihill EE, Drucker DJ (2014) Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev 35:992–1019. https://doi.org/10.1210/er.2014-1035
Fasshauer M, Blüher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36:461–470. https://doi.org/10.1016/j.tips.2015.04.014
Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Müller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J (2011) Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 60:1917–1925. https://doi.org/10.2337/db10-1707
Sell H, Blüher M, Klöting N, Schlich R, Willems M, Ruppe F, Knoefel WT, Dietrich A, Fielding BA, Arner P, Frayn KN, Eckel J (2013) Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care 36:4083–4090. https://doi.org/10.2337/dc13-0496
Zhuge F, Ni Y, Nagashimada M, Nagata N, Xu L, Mukaida N, Kaneko S, Ota T (2016) DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes 65:2966–2979. https://doi.org/10.2337/db16-0317
Shinjo T, Nakatsu Y, Iwashita M, Sano T, Sakoda H, Ishihara H, Kushiyama A, Fujishiro M, Fukushima T, Tsuchiya Y, Kamata H, Nishimura F, Asano T (2015) DPP4 inhibitor anagliptin exerts anti-inflammatory effects on macrophages, adipocytes, and mouse livers by suppressing NF-κB activation. Am J Physiol Endocrinol Metab 309:E214–E223. https://doi.org/10.1152/ajpendo.00553.2014
Drucker DJ (2006) The biology of incretin hormones. Cell Metab 3:153–165. https://doi.org/10.1016/j.cmet.2006.01.004
Gallwitz B (2019) Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 10:389. https://doi.org/10.3389/fendo.2019.00389
Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, Sitagliptin Study 021 Group (2006) Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29:2632–2637. https://doi.org/10.2337/dc06-0703
Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group (2007) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 19:194–205. https://doi.org/10.1111/j.1463-1326.2006.00704.x
Terauchi Y, Yamada Y, Ishida H, Ohsugi M, Kitaoka M, Satoh J, Yabe D, Shihara N, Seino Y (2017) Efficacy and safety of sitagliptin as compared with glimepiride in Japanese patients with type 2 diabetes mellitus aged ≥ 60 years (START-J trial). Diabetes Obes Metab 19:1188–1192. https://doi.org/10.1111/dom.12933
Wada T, Ishikawa A, Watanabe E, Nakamura Y, Aruga Y, Hasegawa H, Onogi Y, Honda H, Nagai Y, Takatsu K, Ishii Y, Sasahara M, Koya D, Tsuneki H, Sasaoka T (2017) Eplerenone prevented obesity-induced inflammasome activation and glucose intolerance. J Endocrinol 235:179–191. https://doi.org/10.1530/JOE-17-0351
Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, Sasahara M, Tsuneki H, Saito S, Sasaoka T (2012) Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab 303:E445–E456. https://doi.org/10.1152/ajpendo.00638.2011
Ishikawa A, Wada T, Nishimura S, Ito T, Okekawa A, Onogi Y, Watanabe E, Sameshima A, Tanaka T, Tsuneki H, Saito S, Sasaoka T (2020) Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PLoS ONE. https://doi.org/10.1371/journal.pone.0230885
Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, Olefsky JM (2014) Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157:1339–1352. https://doi.org/10.1016/j.cell.2014.05.012
Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, Aminuddin A, Senda S, Tsuneyama K, Ikutani M, Watanabe Y, Igarashi Y, Nagai Y, Takatsu K, Koizumi K, Imura J, Goda N, Sasahara M, Matsumoto M, Saeki K, Nakagawa T, Fujisaka S, Usui I, Tobe K (2016) HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 65:3649–3659. https://doi.org/10.2337/db16-0012
Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K (2013) Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 56:1403–1412. https://doi.org/10.1007/s00125-013-2885-1
Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE (2009) Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29:4467–4483. https://doi.org/10.1128/MCB.00192-09
Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR (2011) The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab 14:491–503. https://doi.org/10.1016/j.cmet.2011.08.006
Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, Blüher M, Czech MP, Tabas I (2018) Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 555:673–677. https://doi.org/10.2337/db16-0317
Varin EM, Mulvihill EE, Beaudry JL, Pujadas G, Fuchs S, Tanti JF, Fazio S, Kaur K, Cao X, Baggio LL, Matthews D, Campbell JE, Drucker DJ (2019) Circulating levels of soluble dipeptidyl peptidase-4 are dissociated from inflammation and induced by enzymatic DPP4 inhibition. Cell Metab 29:320–334. https://doi.org/10.1016/j.cmet.2018.10.001
Ziff OJ, Bromage DI, Yellon DM, Davidson SM (2018) Therapeutic strategies utilizing SDF-1α in ischaemic cardiomyopathy. Cardiovasc Res 114:358–367. https://doi.org/10.1093/cvr/cvx203
Yao L, Heuser-Baker J, Herlea-Pana O, Zhang N, Szweda LI, Griffin TM, Barlic-Dicen J (2014) Deficiency in adipocyte chemokine receptor CXCR4 exacerbates obesity and compromises thermoregulatory responses of brown adipose tissue in a mouse model of diet-induced obesity. FASEB J 28:4534–4550. https://doi.org/10.1096/fj.14-249797
Shin J, Fukuhara A, Onodera T, Kita S, Yokoyama C, Otsuki M, Shimomura I (2018) SDF-1 is an autocrine insulin-desensitizing factor in adipocytes. Diabetes 67:1068–1078. https://doi.org/10.2337/db17-0706
Quinn KE, Mackie DI, Caron KM (2018) Emerging roles of atypical chemokine receptor 3 (ACKR3) in normal development and physiology. Cytokine 109:17–23. https://doi.org/10.1016/j.cyto.2018.02.024
Janssens R, Mortier A, Boff D, Ruytinx P, Gouwy M, Vantilt B, Larsen O, Daugvilaite V, Rosenkilde MM, Parmentier M, Noppen S, Liekens S, Van Damme J, Struyf S, Teixeira MM, Amaral FA, Proost P (2018) Truncation of CXCL12 by CD26 reduces its CXC chemokine receptor 4- and atypical chemokine receptor 3-dependent activity on endothelial cells and lymphocytes. Biochem Pharmacol 132:92–101. https://doi.org/10.1016/j.bcp.2017.03.009
Gustavsson M, Dyer DP, Zhao C, Handel TM (2019) Kinetics of CXCL12 binding to atypical chemokine receptor 3 reveal a role for the receptor N terminus in chemokine binding. Sci Signal 12:eaaw3657. https://doi.org/10.1126/scisignal.aaw3657
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number JP24591318 (to T.W.), a research grant from the Sanwa Kagaku Kenkyusho (to T.S. and T.W.), and a research grant from Novartis Pharma Corporation (to T.W.). The authors thank Y. Kurashige and T. Matsushima at the Department of Pathology, and Y. Kotera and Y. Miyazawa at the Department of Clinical Pharmacology, University of Toyama, for their excellent technical assistance.
Funding
This study was supported by JSPS KAKENHI grant number 18K08469 (to T.W.), a research Grant from the Sanwa Kagaku Kenkyusho (to T.S. and T.W.), and a research grant from Novartis Pharma Corporation (to T.W.).
Author information
Authors and Affiliations
Contributions
Conceptualization: TW; Methodology: TW, EW, and YO; Formal analysis and investigation: TW, EW, AO, FK, GK, and YO; Writing—original draft preparation: TW; Writing—review and editing: TW, EW, HT, and TS; Funding acquisition: TW, TS; Supervision: TW, and TS. All the authors shared ideas and contributed to the discussion, and commented on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Watanabe, E., Wada, T., Okekawa, A. et al. Stromal cell-derived factor 1 (SDF1) attenuates platelet-derived growth factor-B (PDGF-B)-induced vascular remodeling for adipose tissue expansion in obesity. Angiogenesis 23, 667–684 (2020). https://doi.org/10.1007/s10456-020-09738-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-020-09738-6