Abstract
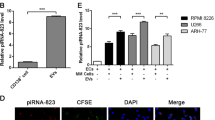
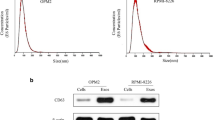
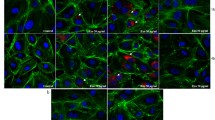
Bone marrow microenvironment is known to support angiogenesis, thus contributing to progression of multiple myeloma (MM). Bortezomib, a proteasome inhibitor (PI) widely used in MM treatment, has anti-angiogenic activity. Extracellular vesicles (EVs), shedding from cell surface, serve as mediators in cell-to-cell communication. We have hypothesized that MM cells (MMCs) treated with bortezomib generate EVs that could diminish angiogenesis, thus limiting MM progression. In the present study, EVs were obtained from MMCs (RPMI-8226), untreated (naïve) or pre-treated with bortezomib. EVs were outlined using NanoSight, FACS, protein arrays and proteasome activity assays. The impact of MMC-EVs on endothelial cell (EC) functions was assessed, employing XTT assay, Boyden chamber and Western blot. A high apoptosis level (annexin V binding 70.25 ± 16.37%) was observed in MMCs following exposure to bortezomib. Compared to naïve EVs, a large proportion of bortezomib-induced EVs (Bi-EVs) were bigger in size (> 300 nm), with higher levels of annexin V binding (p = 0.0043).They also differed in content, presenting with increased levels of pro-inflammatory proteins, reduced levels of pro-angiogenic growth factors (VEGFA, PDGF-BB, angiogenin), and displayed lower proteasome activity. Naïve EVs were found to promote EC migration and proliferation via ERK1/2 and JNK1/2/3 phosphorylation, whereas Bi-EVs inhibited these functions. Moreover, Bi-EVs appeared to reduce EC proteasome activity. EVs released from apoptotic MMCs following treatment with bortezomib can promote angiogenesis suppression by decreasing proliferation and migration of EC. These activities are found to be mediated by specific signal transduction pathways.






Similar content being viewed by others
References
Ria R, Vacca A, Russo F, Cirulli T, Massaia M, Tosi P et al (2004) A VEGF-dependent autocrine loop mediates proliferation and capillarogenesis in bone marrow endothelial cells of patients with multiple myeloma. Thromb Haemost 92:1438–1445
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M et al (2013) BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest 123:1542–1555
Xu S, Menu E, De Becker A, Van Camp B, Vanderkerken K, Van Riet I (2012) Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells 30:266–279
Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM (2012) Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol 2012:157496
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410
Ntellas P, Perivoliotis K, Dadouli K, Koukoulis GK, Ioannou M (2017) Microvessel density as a surrogate prognostic marker in patients with multiple myeloma: a meta-analysis. Acta Haematol 138:77–84
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105
Podar K, Tonon G, Sattler M, Tai YT, Legouill S, Yasui H et al (2006) The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc Natl Acad Sci USA 103:19478–19483
Tu Y, Chen C, Pan J, Xu J, Zhou ZG, Wang CY (2012) The Ubiquitin Proteasome Pathway (UPP) in the regulation of cell cycle control and DNA damage repair and its implication in tumorigenesis. Int J Clin Exp Pathol 5:726–738
Juvekar A, Manna S, Ramaswami S, Chang TP, Vu HY, Ghosh CC et al (2011) Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB-dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res 9:183–194
Pei XY, Dai Y, Grant S (2003) The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia 17:2036–2045
Kubiczkova L, Pour L, Sedlarikova L, Hajek R, Sevcikova S (2014) Proteasome inhibitors—molecular basis and current perspectives in multiple myeloma. J Cell Mol Med 18:947–961
Lynch C, Panagopoulou M, Gregory CD (2017) Extracellular vesicles arising from apoptotic cells in tumors: roles in cancer pathogenesis and potential clinical applications. Front Immunol 8:1174
Choi D, Lee TH, Spinelli C, Chennakrishnaiah S, D’Asti E, Rak J (2017) Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol 67:11–22
Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF et al (2017) A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun 8:14450
Huang Z, Feng Y (2017) Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-induced beta-catenin signaling in endothelial cells. Oncol Res 25:651–661
Vader P, Breakefield XO, Wood MJ (2014) Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med 20:385–393
Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B et al (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68:2667–2688
Tzoran I, Rebibo-Sabbah A, Brenner B, Aharon A (2015) Disease dynamics in patients with acute myeloid leukemia: new biomarkers. Exp Hematol 43:936–943
Aharon A, Sabbah A, Ben-Shaul S, Berkovich H, Loven D, Brenner B et al (2017) Chemotherapy administration to breast cancer patients affects extracellular vesicles thrombogenicity and function. Oncotarget 8:63265–63280
Arendt BK, Walters DK, Wu X, Tschumper RC, Jelinek DF (2014) Multiple myeloma dell-derived microvesicles are enriched in CD147 expression and enhance tumor cell proliferation. Oncotarget 5:5686–5699
Liu Y, Zhu XJ, Zeng C, Wu PH, Wang HX, Chen ZC et al (2014) Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol Sin 35:230–238
Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J et al (2001) The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 61:3071–3076
Aharon A, Brenner B, Katz T, Miyagi Y, Lanir N (2004) Tissue factor and tissue factor pathway inhibitor levels in trophoblast cells: implications for placental hemostasis. Thromb Haemost 92:776–786
Aharon A, Tamari T, Brenner B (2008) Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost 100:878–885
Liao H, He H, Chen Y, Zeng F, Huang J, Wu L (2014) Effects of long-term serial cell passaging on cell spreading, migration, and cell-surface ultrastructures of cultured vascular endothelial cells. Cytotechnology 66:229–238
Katzenell S, Shomer E, Zipori Y, Zylberfisz A, Brenner B, Aharon A (2012) Characterization of negatively charged phospholipids and cell origin of microparticles in women with gestational vascular complications. Thromb Res 130:479–484
Bochmann I, Ebstein F, Lehmann A, Wohlschlaeger J, Sixt SU, Kloetzel PM et al (2014) T lymphocytes export proteasomes by way of microparticles: a possible mechanism for generation of extracellular proteasomes. J Cell Mol Med 18:59–68
Wang J, De Veirman K, Faict S, Frassanito MA, Ribatti D, Vacca A et al (2016) Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol 239:162–173
Mohan M, Matin A, Davies FE (2017) Update on the optimal use of bortezomib in the treatment of multiple myeloma. Cancer Manag Res 9:51–63
Jung L, Holle L, Dalton WS (2004) Discovery, development, and clinical applications of bortezomib. Oncology 18:4–13
Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I et al (2013) Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. https://doi.org/10.3402/jev.v2i0.20677
Koifman N, Biran I, Aharon A, Brenner B, Talmon Y (2017) A direct-imaging cryo-EM study of shedding extracellular vesicles from leukemic monocytes. J Struct Biol 198:177–185
Lasser C, Jang SC, Lotvall J (2018) Subpopulations of extracellular vesicles and their therapeutic potential. Mol Aspects Med 60:1–14
Neumann B, Klippert A, Raue K, Sopper S, Stahl-Hennig C (2015) Characterization of B and plasma cells in blood, bone marrow, and secondary lymphoid organs of rhesus macaques by multicolor flow cytometry. J Leukoc Biol 97:19–30
Turturici G, Tinnirello R, Sconzo G, Geraci F (2014) Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol 306:C621–C633
Martin RK, Brooks KB, Henningsson F, Heyman B, Conrad DH (2014) Antigen transfer from exosomes to dendritic cells as an explanation for the immune enhancement seen by IgE immune complexes. PLoS ONE 9:e110609
Kahn R, Mossberg M, Stahl AL, Johansson K, Lopatko Lindman I, Heijl C et al (2017) Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int 91:96–105
Di Raimondo F, Azzaro MP, Palumbo G, Bagnato S, Giustolisi G, Floridia P et al (2000) Angiogenic factors in multiple myeloma: higher levels in bone marrow than in peripheral blood. Haematologica 85:800–805
Kumar S, Rajkumar SV, Greipp PR, Witzig TE (2004) Cell proliferation of myeloma plasma cells: comparison of the blood and marrow compartments. Am J Hematol 77:7–11
Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F (2017) Extracellular vesicles in angiogenesis. Circ Res 120:1658–1673
Li XM, Zhang LP, Wang YZ, Lu AD, Chang Y, Zhu HH et al (2016) CD38+ CD58− is an independent adverse prognostic factor in paediatric Philadelphia chromosome negative B cell acute lymphoblastic leukaemia patients. Leuk Res 43:33–38
Tsirakis G, Pappa CA, Kanellou P, Stratinaki MA, Xekalou A, Psarakis FE et al (2012) Role of platelet-derived growth factor-AB in tumour growth and angiogenesis in relation with other angiogenic cytokines in multiple myeloma. Hematol Oncol 30:131–136
Coluccia AM, Cirulli T, Neri P, Mangieri D, Colanardi MC, Gnoni A et al (2008) Validation of PDGFRbeta and c-Src tyrosine kinases as tumor/vessel targets in patients with multiple myeloma: preclinical efficacy of the novel, orally available inhibitor dasatinib. Blood 112:1346–1356
Miyake M, Goodison S, Lawton A, Gomes-Giacoia E, Rosser CJ (2015) Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway. Oncogene 34:890–901
Alexandrakis MG, Passam FH, Sfiridaki A, Kyriakou DS, Petreli E, Roussou P (2003) Elevated serum angiogenin in multiple myeloma. Hematol J 4:454–455
Pappa CA, Tsirakis G, Boula A, Sfiridaki A, Psarakis FE, Alexandrakis MG et al (2013) The significance of non correlation between interleukin-8 serum levels with bone marrow microvascular density in patients with myeloma multiple. Pathol Oncol Res 19:539–543
Herkenne S, Paques C, Nivelles O, Lion M, Bajou K, Pollenus T et al (2015) The interaction of uPAR with VEGFR2 promotes VEGF-induced angiogenesis. Sci Signal 8:ra117
Kovacs E (2010) Interleukin-6 leads to interleukin-10 production in several human multiple myeloma cell lines. Does interleukin-10 enhance the proliferation of these cells? Leuk Res 34:912–916
Menu E, De Leenheer E, De Raeve H, Coulton L, Imanishi T, Miyashita K et al (2006) Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis 23:291–300
Bhagat K, Vallance P (1997) Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation 96:3042–3047
Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R et al (2013) The role of IL-1beta in the early tumor cell-induced angiogenic response. J Immunol 190:3500–3509
Ju L, Zhou Z, Jiang B, Lou Y, Guo X (2017) Autocrine VEGF and IL-8 promote migration via Src/Vav2/Rac1/PAK1 signaling in human umbilical vein endothelial cells. Cell Physiol Biochem 41: 1346–1359
Beyar-Katz O, Magidey K, Ben-Tsedek N, Alishekevitz D, Timaner M, Miller V et al (2016) Bortezomib-induced pro-inflammatory macrophages as a potential factor limiting anti-tumour efficacy. J Pathol 239:262–273
Singha B, Gatla HR, Manna S, Chang TP, Sanacora S, Poltoratsky V et al (2014) Proteasome inhibition increases recruitment of IkappaB kinase beta (IKKbeta), S536P-p65, and transcription factor EGR1 to interleukin-8 (IL-8) promoter, resulting in increased IL-8 production in ovarian cancer cells. J Biol Chem 289:2687–2700
Alotaibi MR, Hassan ZK, Al-Rejaie SS, Alshammari MA, Almutairi MM, Alhoshani AR et al (2018) Characterization of apoptosis in a breast cancer cell line after IL-10 silencing. Asian Pac J Cancer Prev (APJCP) 19:777–783
Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P et al (1999) VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res 247:495–504
Wen J, Feng Y, Huang W, Chen H, Liao B, Rice L et al (2010) Enhanced antimyeloma cytotoxicity by the combination of arsenic trioxide and bortezomib is further potentiated by p38 MAPK inhibition. Leuk Res 34:85–92
Edwards CM, Lwin ST, Fowler JA, Oyajobi BO, Zhuang J, Bates AL et al (2009) Myeloma cells exhibit an increase in proteasome activity and an enhanced response to proteasome inhibition in the bone marrow microenvironment in vivo. Am J Hematol 84:268–272
Osawa T, Naito T, Kaneko T, Mino Y, Ohnishi K, Yamada H et al (2014) Blood distribution of bortezomib and its kinetics in multiple myeloma patients. Clin Biochem 47:54–59
Acknowledgements
We would like to thank Mrs. Sonia Kamenetsky for assistance in the preparation of this manuscript.
Funding
This study was funded by the Israel Cancer Association (Grant Number 20120100) and by the Israel myeloma association (AMEN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2018_9649_MOESM1_ESM.pptx
Supplementary material 1 Effects of MMC-EVs on EC signaling and proteasome activity. HUVEC were incubated in the serum-free medium for 16 hours followed by addition of inhibitors U0126, PD98059, SP600125, and SB203580 for 1 hour. EVs generated from the RPMI 8226 cell line (MMC-EVs: naïve-EVs and Bi-EVs) were added to the culture for 15 minutes. Cellular lysates were separated by SDS-PAGE. Phosphorylation and protein levels of (a) ERK1/2, (b) c-Jun, (c) MAPKAPK-2 were assessed using Western blot (equal protein loading was confirmed with β-actin). Phosphorylation levels were normalized to the total protein expression and displayed in gel pictures S1 (a–c) (PPTX 354 KB)
Rights and permissions
About this article
Cite this article
Zarfati, M., Avivi, I., Brenner, B. et al. Extracellular vesicles of multiple myeloma cells utilize the proteasome inhibitor mechanism to moderate endothelial angiogenesis. Angiogenesis 22, 185–196 (2019). https://doi.org/10.1007/s10456-018-9649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-018-9649-y