Abstract
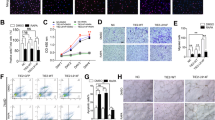
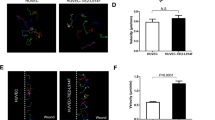
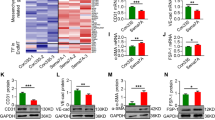
A missense mutation from arginine to tryptophan at residue 849 in the kinase domain of Tie2 (Tie2-R849W) is commonly identified in familial venous malformations. The mechanistic action of Tie2-R849W variant expression on angiogenic cascades including smooth muscle cell recruitment, however, remains elusive. To avoid confounding factors from endogenous Tie2 expression, Tie2-depleted endothelial cells (ECs) were used to study the effects of ectopic shRNA-resistant Tie2 variant expression, Tie2-WT* and Tie2-R849W*, on vascular cell proliferation, migration, tube formation, and smooth muscle cell (SMC) recruitment. Tie2-R849W* induced STAT1 phosphorylation at Tyr701. Tie2-R849W*-expressing cells had reduced ability to migrate and form tubes on Matrigel than their wildtype counterparts. STAT1 phosphorylation attenuated VEGF-A-induced STAT3 tyrosine phosphorylation in Tie2-R849W*-expressing HUVECs. The induced STAT1 activation also decreased VEGF-A-induced bFGF mRNA expression by competing with activated STAT3 for a direct binding to the consensus STAT-binding site at positions −997 to −989 bp from transcription start site in the bFGF promoter. Depleting STAT1 expression rescued the inability of Tie2-R849W expression to mediate angiogenesis. Moreover, bFGF neutralization or constitutive STAT1 activation, reminiscence of Tie2-R849W* expression, suppressed the smooth muscle cell recruiting ability of endothelial conditioned medium. This work reveals an anti-angiogenic role of STAT1 activation that acts in Tie2-R849W-expressing ECs to impair VEGF-A-mediated STAT3 signaling, bFGF production, and smooth muscle cell recruitment. A balancing activity of STAT1 and STAT3 may be important for Tie2-mediated vascular homeostasis.






Similar content being viewed by others
References
Breier G (2000) Angiogenesis in embryonic development–a review. Placenta 21(Suppl A):S11–S15. doi:10.1053/plac.1999.0525
Costa C, Soares R, Schmitt F (2004) Angiogenesis: now and then. APMIS 112(7–8):402–412. doi:10.1111/j.1600-0463.2004.apm11207-0802.x
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J (2000) Vascular-specific growth factor and blood vessel formation. Nature 407:242–248. doi:10.1038/35025215
Suzuki Y, Komi Y, Ashino H, Yamashita J, Inoue J, Yoshiki A, Eichmann A, Amanuma H, Kojima S (2004) Retinoic acid controls blood vessel formation by modulating endothelial and mural cell interaction via suppression of Tie2 signaling in vascular progenitor cells. Blood 104(1):166–169. doi:10.1182/blood-2003-09-3293
Ward NL, Dumont DJ (2002) The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol 13(1):19–27. doi:10.1006/scdb.2001.0288
Fujikawa K, de Aos ScherpenseelI, Jain SK, Presman E, Christensen RA, Varticovski L (1999) Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp Cell Res 253(2):663–672. doi:10.1006/excr.1999.4693
Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC (2000) Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 275(13):9102–9105. doi: 10.1074/jbc.275.13.9102
Conway EM, Collen D, Carmeliet P (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res 49(3):507–521. doi: 10.1016/S0008-6363(00)00281-9
Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y (1995) Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376(6535):70–74. doi: 10.1038/376070a0
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87(7):1171–1180. doi: 10.1016/S0092-8674(00)81813-9
Loughna S, Sato TN (2001) Angiopoietin and Tie signaling pathways in vascular development. Matrix Biol 20(5–6):319–325. doi: 10.1016/S0945-053X(01)00149-4
Vikkula M, Boon LM, Carraway KL III, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR (1996) Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 87(7):1181–1190. doi: 10.1016/S0092-8674(00)81814-0
Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, Eklund L, Boon LM, Vikkula M (2009) Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet 41(1):118–124. doi:10.1038/ng.272
Wouters V, Limaye N, Uebelhoer M, Irrthum A, Boon LM, Mulliken JB, Enjolras O, Baselga E, Berg J, Dompmartin A, Ivarsson SA, Kangesu L, Lacassie Y, Murphy J, Teebi AS, Penington A, Rieu P, Vikkula M (2010) Hereditary cutaneomucosal venous malformations are caused by TIE2 mutations with widely variable hyper-phosphorylating effects. Eur J Hum Genet 18(4):414–420. doi:10.1038/ejhg.2009.193
Morris PN, Dunmore BJ, Tadros A, Marchuk DA, Darland DC, D’Amore PA, Brindle NP (2005) Functional analysis of a mutant form of the receptor tyrosine kinase Tie2 causing venous malformations. J Mol Med 83(1):58–63. doi:10.1007/s00109-004-0601-9
Morris PN, Dunmore BJ, Brindle NP (2006) Mutant Tie2 causing venous malformation signals through Shc. Biochem Biophys Res Commun 346(1):335–338. doi:10.1016/j.bbrc.2006.05.128
Korpelainen EI, Karkkainen M, Gunji Y, Vikkula M, Alitalo K (1999) Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant Tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene 18(1):1–8. doi:10.1038/sj.onc.1202288
Hu HT, Huang YH, Chang YA, Lee CK, Jiang MJ, Wu LW (2008) Tie2-R849W mutant in venous malformations chronically activates a functional STAT1 to modulate gene expression. J Invest Dermatol 128(9):2325–2333. doi:10.1038/jid.2008.89
Wincewicz A, Sulkowska M, Rutkowski R, Sulkowski S, Musiatowicz B, Hirnle T, Famulski W, Koda M, Sokol G, Szarejko P (2007) STAT1 and STAT3 as intracellular regulators of vascular remodeling. Eur J Intern Med 18(4):267–271. doi:10.1016/j.ejim.2006.12.007
Huang S, Bucana CD, Van Arsdall M, Fidler IJ (2002) STAT1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene 21(16):2504–2512. doi:10.1038/sj.onc.1205341
Kim JY, Bae YH, Bae MK, Kim SR, Park HJ, Wee HJ, Bae SK (2009) Visfatin through STAT3 activation enhances IL-6 expression that promotes endothelial angiogenesis. Biochim Biophys Acta 1793(11):1759–1767. doi:10.1016/j.bbamcr.2009.09.006
Brantley EC, Nabors LB, Gillespie GY, Choi YH, Palmer CA, Harrison K, Roarty K, Benveniste EN (2008) Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res 14(15):4694–4704. doi:10.1158/1078-0432.CCR-08-0618
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H (2002) Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21(13):2000–2008. doi:10.1038/sj.onc.1205260
Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S (2006) Activation of STAT3 in human melanoma promotes brain metastasis. Cancer Res 66(6):3188–3196. doi:10.1158/0008-5472.CAN-05-2674
Jih YJ, Lien WH, Tsai WC, Yang GW, Li C, Wu LW (2001) Distinct regulation of genes by bFGF and VEGF-A in endothelial cells. Angiogenesis 4:313–321. doi: 10.1023/A:1016080321956
Leik CE, Willey A, Graham MF, Walsh SW (2004) Isolation and culture of arterial smooth muscle cells from human placenta. Hypertension 43(4):837–840. doi:10.1161/01.HYP.0000119191.33112.9c
Sironi JJ, Ouchi T (2004) STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J Biol Chem 279(6):4066–4074. doi:10.1074/jbc.M307774200
Zhang Q, Wang HY, Woetmann A, Raghunath PN, Odum N, Wasik MA (2006) STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood 108(3):1058–1064. doi:10.1182/blood-2005-08-007377
Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY (1999) Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res 58(3):224–237. doi: 10.1006/mvre.1999.2179
Bogdanovic E, Nguyen VP, Dumont DJ (2006) Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J Cell Sci 119(Pt 17):3551–3560. doi:10.1242/jcs.03077
Coultas L, Chawengsaksophak K, Rossant J (2005) Endothelial cells and VEGF in vascular development. Nature 438(7070):937–945. doi:10.1038/nature04479
Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, Detmar M, Hashimoto K (2003) Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem 278(41):40026–40031. doi:10.1074/jbc.M301866200
Chen Z, Han ZC (2008) STAT3: a critical transcription activator in angiogenesis. Med Res Rev 28(2):185–200. doi:10.1002/med.20101
Wen Z, Zhong Z, Darnell JE Jr (1995) Maximal activation of transcription by STAT1 and STAT3 requires both tyrosine and serine phosphorylation. Cell 82(2):241–250. doi:10.1016/0092-8674(95)90311-9
Battle TE, Lynch RA, Frank DA (2006) Signal transducer and activator of transcription 1 activation in endothelial cells is a negative regulator of angiogenesis. Cancer Res 66(7):3649–3657. doi:10.1158/0008-5472.CAN-05-3612
Yeh HH, Lai WW, Chen HH, Liu HS, Su WC (2006) Autocrine IL-6-induced STAT3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 25(31):4300–4309. doi:10.1038/sj.onc.1209464
Pine R, Canova A, Schindler C (1994) Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J 13(1):158–167
Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP (2007) Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27(8):1736–1743. doi:10.1161/ATVBAHA.107.142117
Findley CM, Cudmore MJ, Ahmed A, Kontos CD (2007) VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol 27(12):2619–2626. doi:10.1161/ATVBAHA.107.150482
Singh H, Hansen TM, Patel N, Brindle NP (2012) The molecular balance between receptor tyrosine kinases Tie1 and Tie2 is dynamically controlled by VEGF and TNFalpha and regulates angiopoietin signalling. PLoS ONE 7(1):e29319. doi:10.1371/journal.pone.0029319
Harris AL, Reusch P, Barleon B, Hang C, Dobbs N, Marme D (2001) Soluble Tie2 and Flt1 extracellular domains in serum of patients with renal cancer and response to antiangiogenic therapy. Clin Cancer Res 7(7):1992–1997
Stephanou A (2004) Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med 8(4):519–525. doi: 10.1111/j.1582-4934.2004.tb00476.x
Stephanou A, Latchman DS (2005) Opposing actions of STAT-1 and STAT-3. Growth Factors 23(3):177–182. doi:10.1080/08977190500178745
Qing Y, Stark GR (2004) Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem 279(40):41679–41685. doi:10.1074/jbc.M406413200
Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is’harc H, Gesualdo I, Newman SJ, Kerr IM, Poli V (2002) Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Nat Acad Sci USA 99(12):8043–8047. doi:10.1073/pnas.122236099
Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li D, Wu Y, Song Y, Luo J, Pang X, Yi Z, Liu M (2010) Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 31(12):2097–2104. doi:10.1093/carcin/bgq167
Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G (2006) Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 107(7):2705–2712. doi:10.1182/blood-2005-09-3541
Megeney LA, Perry RL, LeCouter JE, Rudnicki MA (1996) bFGF and LIF signaling activates STAT3 in proliferating myoblasts. Dev Genet 19(2):139–145. doi:10.1002/(SICI)1520-6408(1996)19:2<139:AID-DVG5>3.0.CO;2-A
Li D, Zhang C, Song F, Lubenec I, Tian Y, Song QH (2009) VEGF regulates FGF-2 and TGF-beta1 expression in injury endothelial cells and mediates smooth muscle cells proliferation and migration. Microvasc Res 77(2):134–142. doi:10.1016/j.mvr.2008.09.007
Cucina A, Borrelli V, Randone B, Coluccia P, Sapienza P, Cavallaro A (2003) Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surg Res 109(1):16–23. doi: 10.1016/S0022-4804(02)00042-2
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9(6):685–693. doi:10.1038/nm0603-685
Acknowledgments
This work was supported by National Science Council (98-2320-B-006-034-MY3, 99-2628-B-006-017-MY3, and 100-2325-B006-005) and Department of Health (DOH-101-TD-C-111-003) to Wu LW. A fellowship for establishing centers of excellence for cancer research from Department of Health, Executive Yuan in Taiwan (DOH100-TD-C-111-003) was awarded to Huang YH. All shRNA plasmids were obtained from the National RNAi Core Facility at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by the National Research Program for Genomic Medicine Grants by National Science Council (NSC 97-B-3112-B-001-016). All the authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, YH., Wu, MP., Pan, SC. et al. STAT1 activation by venous malformations mutant Tie2-R849W antagonizes VEGF-A-mediated angiogenic response partly via reduced bFGF production. Angiogenesis 16, 207–222 (2013). https://doi.org/10.1007/s10456-012-9313-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9313-x