Abstract
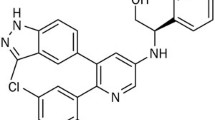
Small molecular inhibitors of Cyclin dependent kinases (Cdks) are currently being developed as anticancer therapeutics due to their antiproliferative properties. The purine Cdk specific inhibitor (R)-roscovitine (seliciclib, CYC202) represents one of the most promising of these compounds. It is currently evaluated in clinical trials concerning cancer therapy. Recently, we have shown that roscovitine exerts potent antiangiogenic effects and elucidated Cdk5 as a new player in angiogenesis. These findings introduce Cdk5 as novel target for antiangiogenic therapy, and Cdk5 inhibitors as an attractive therapeutic approach. Here, we present the antiangiogenic profile of 15 derivatives of roscovitine in vitro and in vivo and provide structure activity relationships of the roscovitine analogs. The (S)-isomer LGR561 and the respective (R)- and (S)-isomers LGR848 and LGR849 strongly inhibited proliferation and cell cycle progression, induced cell death, and reduced migration of endothelial cells in vitro. In comparison to roscovitine, these compounds showed an increased potency to inhibit Cdk2, Cdk5, Cdk7, and Cdk9. By analyzing the effects of LGR561, LGR848, and LGR849 on endothelial cell tube formation, mouse aortic ring sprouting, angiogenesis in the chick chorioallantoic membrane, and neovessel formation in the mouse cornea, we elucidate the two (S)-isomers LGR561 and LGR849 as highly potent inhibitors of angiogenesis. This study provides first information on how to modify roscovitine to develop Cdk inhibitors with increased antiangiogenic activity and suggests the application of existing and the development of new Cdk inhibitors to inhibit both, cancer cell proliferation and angiogenesis.




Similar content being viewed by others
References
Senderowicz AM (2003) Small-molecule cyclin-dependent kinase modulators. Oncogene 22:6609–6620
Senderowicz AM (2004) Targeting cell cycle and apoptosis for the treatment of human malignancies. Curr Opin Cell Biol 16:670–678
Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP (1997) Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 243:527–536
Legraverend M, Ludwig O, Bisagni E, Leclerc S, Meijer L, Giocanti N, Sadri R, Favaudon V (1999) Synthesis and in vitro evaluation of novel 2, 6, 9-trisubstituted purines acting as cyclin-dependent kinase inhibitors. Bioorg Med Chem 7:1281–1293
Krystof V, Lenobel R, Havlicek L, Kuzma M, Strnad M (2002) Synthesis and biological activity of olomoucine II. Bioorg Med Chem Lett 12:3283–3286
Krystof V, McNae IW, Walkinshaw MD, Fischer PM, Muller P, Vojtesek B, Orsag M, Havlicek L, Strnad M (2005) Antiproliferative activity of olomoucine II, a novel 2, 6, 9-trisubstituted purine cyclin-dependent kinase inhibitor. Cell Mol Life Sci 62:1763–1771
Aldoss IT, Tashi T, Ganti AK (2009) Seliciclib in malignancies. Expert Opin Investig Drugs 18:1957–1965
Guzi T (2004) CYC-202 Cyclacel. Curr Opin Investig Drugs 5:1311–1318
Tonini T, Rossi F, Claudio PP (2003) Molecular basis of angiogenesis and cancer. Oncogene 22:6549–6556
Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8:592–603
Abadi AH, Abou-Seri SM, Abdel-Rahman DE, Klein C, Lozach O, Meijer L (2006) Synthesis of 3-substituted-2-oxoindole analogues and their evaluation as kinase inhibitors, anticancer and antiangiogenic agents. Eur J Med Chem 41:296–305
Maggiorella L, Aubel C, Haton C, Milliat F, Connault E, Opolon P, Deutsch E, Bourhis J (2009) Cooperative effect of roscovitine and irradiation targets angiogenesis and induces vascular destabilization in human breast carcinoma. Cell Prolif 42:38–48
Zahler S, Liebl J, Furst R, Vollmar AM (2010) Anti-angiogenic potential of small molecular inhibitors of cyclin dependent kinases in vitro. Angiogenesis
Liebl J, Weitensteiner SB, Vereb G, Takacs L, Füerst R, Vollmar AM, Zahler S (2010) Cyclin dependent kinase 5 (Cdk5) regulates endothelial cell migration and angiogenesis. J Biol Chem
Havlicek L, Hanus J, Vesely J, Leclerc S, Meijer L, Shaw G, Strnad M (1997) Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J Med Chem 40:408–412
Otyepka M, Krystof V, Havlicek L, Siglerova V, Strnad M, Koca J (2000) Docking-based development of purine-like inhibitors of cyclin-dependent kinase-2. J Med Chem 43:2506–2513
Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L et al (1998) Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281:533–538
Kiemer AK, Weber NC, Fürst R, Bildner N, Kulhanek-Heinze S, Vollmar AM (2002) Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res 90:874–881
Koltermann A, Liebl J, Fürst R, Ammer H, Vollmar AM, Zahler S (2009) Ginkgo biloba extract EGb 761 exerts anti-angiogenic effects via activation of tyrosine phosphatases. J Cell Mol Med 13:2122–2130
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279
Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ (1996) A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 37:1625–1632
Licht T, Tsirulnikov L, Reuveni H, Yarnitzky T, Ben-Sasson SA (2003) Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3). Blood 102:2099–2107
De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH (1997) Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem 243:518–526
Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, Koken M, Coburn SP, Tang L, Jiang T et al (2005) Roscovitine targets, protein kinases and pyridoxal kinase. J Biol Chem 280:31208–31219
Menn B, Bach S, Blevins TL, Campbell M, Meijer L, Timsit S (2010) Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo CDK5 activity increase in animal stroke models. PLoS One 5:e12117
Feldmann G, Mishra A, Hong SM, Bisht S, Strock CJ, Ball DW, Goggins M, Maitra A, Nelkin BD (2010) Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res 70:4460–4469
Strock CJ, Park JI, Nakakura EK, Bova GS, Isaacs JT, Ball DW, Nelkin BD (2006) Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res 66:7509–7515
Liu JL, Wang XY, Huang BX, Zhu F, Zhang RG, Wu G (2010) Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med Oncol
Liu J, Zhang DL, Shan SG (2008) Expression and clinical significance of cyclin H and CDK7 in human hemangiomas. Zhonghua Zheng Xing Wai Ke Za Zhi 24:300–302
Wagner N, Jehl-Pietri C, Lopez P, Murdaca J, Giordano C, Schwartz C, Gounon P, Hatem SN, Grimaldi P, Wagner KD (2009) Peroxisome proliferator-activated receptor beta stimulation induces rapid cardiac growth and angiogenesis via direct activation of calcineurin. Cardiovasc Res 83:61–71
Ali S, Heathcote DA, Kroll SH, Jogalekar AS, Scheiper B, Patel H, Brackow J, Siwicka A, Fuchter MJ, Periyasamy M et al (2009) The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Res 69:6208–6215
Fisher RP (2005) Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci 118:5171–5180
Pirngruber J, Shchebet A, Johnsen SA (2009) Insights into the function of the human P-TEFb component CDK9 in the regulation of chromatin modifications and co-transcriptional mRNA processing. Cell Cycle 8:3636–3642
Krystof V, Uldrijan S (2010) Cyclin-dependent kinase inhibitors as anticancer drugs. Curr Drug Targets 11:291–302
Acknowledgments
We thank C. Niemann, H. Stöcker, J. Peliskova, and S. Schnegg for their help with the in vitro models. We thank ProQinase (Freiburg, Germany) for performing in vitro Cdk activity assays. The EU-project PROKINASE No 503467 is gratefully acknowledged. M. Strnad and V. Krystof were supported by projects MSM6198959216 and GACR204/08/0511.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liebl, J., Krystof, V., Vereb, G. et al. Anti-angiogenic effects of purine inhibitors of cyclin dependent kinases. Angiogenesis 14, 281–291 (2011). https://doi.org/10.1007/s10456-011-9212-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-011-9212-6