Abstract
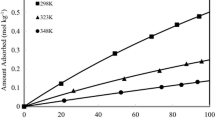
High-silica CHA-type zeolite was synthesized in fluoride medium using fumed silica as silica source. Adsorption isotherms of CO2 and CH4 were measured over pressure range of 0–1100 kPa and at temperatures of 298, 323, 353, and 393 K. The isotherms follow a typical Type-I shape according to the BDDT classification. All adsorption isotherms are well described by Langmuir, Toth and Sips isotherms. Adsorptive performance of the zeolite was compared with other CHA-type zeolites, i.e. SAPO-34, Si-CHA and SSZ-13 using ideal adsorption solution theory. The modeling results revealed that the zeolite has a high CO2/CH4 selectivity, e.g. 6.70 for an equimolar mixture at 298 K and 100 kPa. Isosteric heats of adsorption for both CO2 and CH4 were reasonably constant (20.6 and 24.1 kJ mol−1 at loading of 0.02 mol kg−1 for CH4 and CO2, respectively) showing that the adsorbent is energetically homogenous.









Similar content being viewed by others
Abbreviations
- b:
-
Affinity parameter of Langmuir, Sip and Toth isotherms (kPa− 1)
- Cμs :
-
Saturation adsorption capacity (mol kg− 1)
- Cμ :
-
Amount adsorbed (mol kg− 1)
- k1 :
-
Constant of temperature dependent saturation capacity in Eq. 5 (mol kg− 1)
- k2 :
-
Constant of temperature dependent saturation capacity in Eq. 5 (mol kg− 1 K− 1)
- n:
-
Heterogeneity parameter in Sips isotherm (dimensionless)
- p:
-
Pressure (kPa)
- \({\text{Q}}^{\text{st}}\) :
-
Isosteric heat of adsorption (kJ mol− 1)
- R:
-
Universal gas constant (kJ mol− 1 K− 1)
- R2 :
-
Coefficient of determination (dimensionless)
- r:
-
Correlation coefficient (dimensionless)
- T:
-
Temperature (K)
- t:
-
Heterogeneity parameter in Toth isotherm (dimensionless)
References
Camblor, M.A., Díaz-Cabañas, M.-J., Cox, P.A., Shannon, I.J., Wright, P.A., Morris, R.E.: A synthesis, MAS NMR, synchrotron X-ray powder diffraction, and computational study of zeolite SSZ-23. Chemistry of materials. 11(10), 2878–2885 (1999a)
Camblor, M.A., Villaescusa, L.A., Diaz-Cabanas, M.: Synthesis of all-silica and high-silica molecular sieves in fluoride media. Top. Catal. 9(1), 59–76 (1999b)
Díaz-Cabañas, M.-J., Barrett, P.A.: Synthesis and structure of pure SiO2 chabazite: the SiO2 polymorph with the lowest framework density. Chem. Commun. 17, 1881–1882 (1998)
Do, D.D.: Adsorption analysis: equilibria and kinetics:(With CD Containing Computer Matlab Programs), vol. 2. World Scientific, (1998)
Graham, C., Pierrus, J., Raab, R.: Measurement of the electric quadrupole moments of CO2, CO and N2. Mol. Phys. 67(4), 939–955 (1989)
Himeno, S., Tomita, T., Suzuki, K., Yoshida, S.: Characterization and selectivity for methane and carbon dioxide adsorption on the all-silica DD3R zeolite. Microporous Mesoporous Mater. 98(1), 62–69 (2007)
Hudson, M.R., Queen, W.L., Mason, J.A., Fickel, D.W., Lobo, R.F., Brown, C.M.: Unconventional, highly selective CO2 adsorption in zeolite SSZ-13. J. Am. Chem. Soc. 134(4), 1970–1973 (2012)
IZA. http://www.iza-structure.org/databases/ (2017). Accessed May 2017
Jee, S.E., Sholl, D.S.: Carbon dioxide and methane transport in DDR zeolite: insights from molecular simulations into carbon dioxide separations in small pore zeolites. J. Am. Chem. Soc. 131(22), 7896–7904 (2009)
Jiang, Q., Rentschler, J., Sethia, G., Weinman, S., Perrone, R., Liu, K.: Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process. Chem. Eng. J. 230, 380–388 (2013)
Lide, D., Haynes, W.: CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data-/editor-in-chief, David R. Lide; ass. ed. WM” Mickey” Haunes. CRC, Boca Raton (2009)
Luo, Y., Funke, H.H., Falconer, J.L., Noble, R.D.: Adsorption of CO2, CH4, C3H8, and H2O in SSZ-13, SAPO-34, and T-Type Zeolites. Ind. Eng. Chem. Res. 55(36), 9749–9757 (2016)
Maghsoudi, H.: Equilibrium adsorption analysis of microporous adsorbents in propene/propane binary mixture separation. Adsorption 21(6–7), 547–556 (2015)
Maghsoudi, H.: Comparative study of adsorbents performance in ethylene/ethane separation. Adsorption 22(7), 985–992 (2016)
Maghsoudi, H., Soltanieh, M.: Simultaneous separation of H2S and CO2 from CH4 by a high silica CHA-type zeolite membrane. J. Membr. Sci. 470, 159–165 (2014)
Maghsoudi, H., Soltanieh, M., Bozorgzadeh, H., Mohamadalizadeh, A.: Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 19(5), 1045–1053 (2013)
NIST. http://webbook.nist.gov/chemistry/fluid/ (2017). Accessed Jan 2017
Pham, T.D., Liu, Q., Lobo, R.F.: Carbon dioxide and nitrogen adsorption on cation-exchanged SSZ-13 zeolites. Langmuir. 29(2), 832–839 (2012)
Prodinger, S., Vemuri, R.S., Varga, T., McGrail, B.P., Motkuri, R.K., Derewinski, M.A.: Impact of chabazite SSZ-13 textural properties and chemical composition on CO2 adsorption applications. New J. Chem. 40(5), 4375–4385 (2016)
Rad, M.D., Fatemi, S., Mirfendereski, S.M.: Development of T type zeolite for separation of CO2 from CH4 in adsorption processes. Chem. Eng. Res. Des. 90(10), 1687–1695 (2012)
Talesh, S.S.A., Fatemi, S., Hashemi, S., Ghasemi, M.: Effect of Si/Al ratio on CO2–CH4 adsorption and selectivity in synthesized SAPO-34. Sep. Sci. Technol. 45(9), 1295–1301 (2010)
Yang, J., Li, J., Wang, W., Li, L., Li, J.: Adsorption of CO2, CH4, and N2 on 8-, 10-, and 12-membered ring hydrophobic microporous high-silica zeolites: DDR, silicalite-1, and beta. Ind. Eng. Chem. Res. 52(50), 17856–17864 (2013)
Acknowledgements
The financial support provided by the National Iranian Gas Company is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pourmahdi, Z., Maghsoudi, H. Adsorption isotherms of carbon dioxide and methane on CHA-type zeolite synthesized in fluoride medium. Adsorption 23, 799–807 (2017). https://doi.org/10.1007/s10450-017-9894-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-017-9894-1