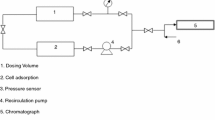
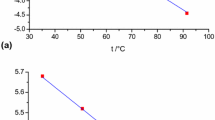
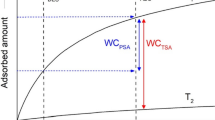
Abstract
The absolute adsorption isotherms are necessary to correctly evaluate the selectivity of the adsorbent material or to design adsorption processes at high pressure (e.g., H2 purification from syngas processes, removal of acid gas from natural gas,…). The aim of this work is thus to propose an easy method to correct the buoyancy effect of the bulk phase on the adsorbed phase volume during both pure gas and gas mixtures adsorption for pressures up to 10 MPa. The potential theory of adsorption and the Dubinin–Radushkevich relation are adapted by introducing mixing parameters based on simple Berthelot rules. The concept of internal pressure used to characterize the adsorbed phase is also adapted for mixtures. The method is then improved on a commercial activated carbon (AC), when adsorbing pure H2S and CH4, and their mixtures up to 5 MPa. The study points out the importance to carefully consider the buoyancy effect of the bulk phase on the adsorbed phase volume. Its impact on the adsorbent material selectivity at high pressures could affect the design and the performances of PSA or TSA processes. For example, only considering the excess adsorption data leads to an apparent selectivity 13 % greater than the absolute one for a concentration of 6 ppm of H2S in a CH4 matrix at 5 MPa (298 K) on the AC.












Similar content being viewed by others
References
Agarwal, R.K., Schwarz J.A.: Analysis of high pressure adsorption of gases on activated carbon by potential theory. Carbon 26, 873–887 (1988)
Berezin, G.I.: Relation between critical parameters of gases and their adsorption constants. Dokl. Akad. Nauk. SSSR 217, 843–845 (1979)
Berezin, G.I.: Calculation of the Henry constant from the critical parameters of an adsorbed gas. Russ. J. Phys. Chem. 57, 391–395 (1983)
Billemont, P., Coasne, B., De Weireld, G.: An experimental and molecular simulation study of the adsorption of carbon dioxide and methane in nanoporous carbons in the presence of water. Langmuir 27, 1015–1024 (2011)
Cook, W.H., Basmadjian, D.: Correlation of adsorption equilibria of pure gases on activated carbon. Can. J. Chem. Eng. 42, 146–151 (1964)
De Weireld, G., Frère, M., Jadot, R.: A new gravimetric method for the determination of high temperature and high pressure gas adsorption isotherms. Meas. Sci. Technol. 10, 117–126 (1999)
De Weireld, G.: Apport expérimental et théeorique à la prédiction du comportement adsorbat/adsorbant dans une large gamme de température et de pression. PhD thesis, Faculté Polytechnique de Mons, Mons, Belgium (2000)
Dreisbach, F., Staudt, R., Tomalla, M., Keller, J.U.: Measurement of adsorption equilibria of pure and mixed corrosive gases: the magnetic suspension balance, In: LeVan, M.D. (ed) Fundamentals of Adsorption. pp 259–268, Kluwer Academic Publishers, Boston (1996)
Dubinin, M.M., Radushkevich, L.V.: Equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. Phys. Chem. Sect. USSR 55, 331–333 (1947).
Dubinin, M.M., Tomofeyev, P.: Adsorption of vapors on active carbons in relation to the properties of the adsorbate. Dokl. Akad. Nauk. SSSR 54, 701–704 (1946)
Dubinin, M.M., Zaverina, E.D.: Adsorption of gases by activated carbon. Dokl. Akad. Nauk. SSSR 72 319 (1950)
Dubinin, M.M., Zaverina, E.D., Radushkevich, L.V.: Sorption and structure of activated carbon. I. Adsorption of organic vapors. Zh. Fiz. Khim. 21, 1351–1362 (1947)
Dubinin, M.M., Neimark, A.V., Sperpinsky, V.V.: Impact of the adsorbate compressibility on the calculation of the micropore volume. Carbon 31 1015–1018 (1993)
Dubinin, M.M.: The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 60, 235–241 (1960)
Dubinin, M.M.: Adsorption in micropores. J. Colloid Interface Sci. 23, 487–499 (1967)
Dubinin, M.M.: Physical adsorption of gases and vapors in micropores. In: Cahenhead DA (ed) Progress in Surface and Membrane Science, pp 1–70. Academic Press, New York (1975)
Frère, M., Jadot, R., Bougard, J.: Determination of the micropore volume distribution function of activated carbons by gas adsorption. Adsorption 3, 55–65 (1996)
Gumma, S., Talu, O.: Net adsorption: A thermodynamic framework for supercritical gas adsorption and storage in porous solids. Langmuir 26, 17013–17023 (2010)
Hamon, L., Frère, M., De Weireld, G.: Development of a new apparatus for gas mixture adsorption measurements coupling gravimetric and chromatographic techniques. Adsorption 14, 493–499 (2008)
Jagiello, J., Schwarz, J.: Relationship between energetic and structural heterogeneity of microporous carbons determined on the basis of adsorption potentials in model micropores. Langmuir 9, 2513–2517 (1993)
Jagiello, J., Bandosz, T., Putyera, K., Schwarz, J.: Adsorption energy and structural heterogeneity of activated carbon. Elsevier Science PBV, Amsterdam (1994)
Krishna, R.: Adsorptive separation of CO2/CH4/CO gas mixtures. Microporous Mesoporous Mater. 156, 217–223 (2012)
Kunz, O., Klimeck, R., Wagner, W., Jaeschke, M.: The GERG-2004 wide-range equation of state for natural gases and other mixtures. VDI Verlag GmbH, Dsseldorf, Germany (2007)
Lemmon, E.W., Span, R., Short fundamental equations of state for 20 industrial fluids. J. Chem. Eng. Data 51, 785–850 (2006)
Lewis, W.K., Gilliland, E.R., Chertow, R., Cadogan, W.P.: Adsorption equilibria. Hydrocarbon gas mixtures. Ind. Eng. Chem. 42, 1319–1326 (1950)
London, F.: Zur Theorie und Systematik der Molekularkräfte, Z. Physik 63, 245–279 (1930)
McCarty, R., Arp, V.: A new wide range of equation of state for helium. Adv. Cryog. Eng. 35, 1465–1475 (1990)
Mehta, S., Danner, R.: An improved potential-theory method for predicting gas-mixture adsorption equilibria, Ind. Eng. Chem. Fundam. 24, 325–330 (1985)
Monsalvo, M.A., Shapiro, A.A.: Modeling adsorption of binary and ternary mixtures on microporous media. Fluid Phase Equilib. 254, 91–100 (2007)
Monsalvo, M.A., Shapiro, A.A.: Study of high-pressure adsorption from supercritical fluids by the potential theory. Fluid Phase Equilib. 283, 56–64 (2009)
Murata, K., El-Merraoui, M., Kaneko, K.: A new determination method of absolute adsorption isotherm of supercritical gases under high pressure with a special relevance to density-functional theory study. J. Chem. Phys. 114, 4196–4205 (2001)
Murata, K., Miyawaki, J., Kaneko, K.: A simple determination method of the absolute adsorbed amount for high pressure gas adsorption. Carbon 40, 425–428 (2002)
Myers, A.L., Monson, P.A.: Adsorption in Porous Materials at High Pressure:? Theory Exp. Langmuir 18, 10261–10273 (2002)
Neimark, A.V.: Potential theory of adsorption and adsorbate compressibility. J. Colloid Interface Sci. 165, 91–96 (1994)
Olney, T., Cann, N., Cooper, G., Brion, C.: Absolute scale determination for photoabsorption spectra and the calculation of molecular properties using dipole sum rules. Chem. Phys. 223, 59–98 (1997)
Ozawa, S., Kusumi, S., Ogino, Y.: Physical adsorption of gases at high pressure. IV. An improvement of the Dubinin–Astakhov adsorption equation. J. Colloid Interface Sci. 56, 83–91 (1976)
Polányi, M.: Über die Adsorption vom Standpunkt des dritten Wärmesatzes. Verh. Dtsch. Phys. Ges. 16, 1012 (1914)
Polányi, M.: Adsorption von Gasen (Dampfen) durch ein festes nichtflüichtiges Adsorberis. Verh. Dtsch. Phys. Ges. 18, 55 (1916)
Polányi, M.: Adsorption aus Lösungen beschränkt löslicher Stoffe. Z. Phys. 2, 111 (1920)
Polányi, M.: Theories of adsorption of gases. General survey and some additional remarks. Trans. Faraday Soc. 28, 316 (1932)
Prausnitz, J.M., Lichtenthaler, R.N., de Azevedo, E.G.: Molecular Thermodynamics of Fluid-Phase Equilibria. Prentice-Hall Inc., Englewood Cliffs, New Jersey (1986)
Ross, D.J.K., Bustin, R.M.: Impact of mass balance calculations on adsorption capacities in microporous shale gas reservoirs. Fuel 86, 2696–2706 (2007)
Rouquerol, F., Rouquerol, J., Sing, K.: Adsorption by Powders and Porous Solids. Academic Press, London, San Diego (1999)
Ruthven, D.M., Farooq, S., Knaebel, K.S.: Pressure swing adsorption. VCH Publishers, New York (1994)
Setzmann, U., Wagner, W.: A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 20, 1061–1151 (1991)
Sircar, S., Golden, T.C.: Pressure swing adsorption technology for hydrogen production. In: Liu, K., Song, C., Subramani, V. (eds) Hydrogen and Syngas Production and Purification Technologies, pp. 414-450. Wiley, Hoboken (2010)
Sircar, S.: Gibbsian surface excess for gas adsorption: revisited. Ind. Eng. Chem. Res. 38, 3670–3682 (1999)
Stoeckli, F., Morel, D.: On the physical meaning of parameters E0 and beta in Dubinin theory. Chimia 34, 502–503 (1980)
Talu, O.: Net adsorption of gas/vapor mixtures in microporous solids. J. Phys. Chem. C 117, 13059–13071 (2013)
Toth, J.: State equations of the solid-phase interface layers, Acta Chim. Acad. Sci. Hungar. 69, 311–328 (1971)
Wood, G.O.: Affnity coeffcients of the Polanyi/Dubinin adsorption isotherm equations. A review with compilations and correlations. Carbon 39, 343–356 (2001)
Acknowledgments
L. Hamon thanks Dr. G. Pirngruber for its advices. L. Hamon and G. De Weireld thank Ir P. Billemont and Dr. N. Heymans for their suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamon, L., Chenoy, L. & De Weireld, G. Determination of absolute gas adsorption isotherms: simple method based on the potential theory for buoyancy effect correction of pure gas and gas mixtures adsorption. Adsorption 20, 397–408 (2014). https://doi.org/10.1007/s10450-013-9579-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9579-3