Abstract
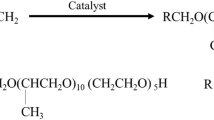
The selective adsorption of the components of a polydisperse gemini surfactant blend (alkylbenzenesulfonate-Jeffamine salt, ABSJ) in aqueous solution onto Berea sandstone, a reference material in enhanced oil recovery (EOR), was investigated. The individual adsorption isotherms of the four, benzene-ring containing ABSJ components with different alkyl chain lengths (ranging from decyl to tridecyl of the alkyl chain length) were simultaneously determined by using a four-channel electrospay ionization mass spectrometer (ESI-MS) for concentration analysis. This analytical device provided selective information (based on the differences in the mass to charge ratio) on the adsorption of each component in the mixed surfactant system. The overall isotherm obtained from the superposition of the individual isotherms determined by ESI-MS agreed well with the isotherm determined by UV spectrometry; the UV equipment is benzene-ring sensitive, irrespective of the alkyl chain length. The S-shaped isotherms reached a plateau at the critical micelle concentration. Longer-chain surfactants adsorbed preferentially over the short chain homologs, independently of solution concentration. This analytical device provided the net adsorption isotherm. Most analytical methods are not component selective, and thus they are not able to measure the individual isotherms in multicomponent solutions. Here, we report on a novel method which describes the selective determination of the individual adsorption isotherms of surfactants in a multicomponent mixture. The theoretical background of the method is described in detail.







Similar content being viewed by others
References
Curbelo, F.D.S., Santanna, V.C., Neto, E.L.B., Dutra, T.V., Dantas, T.N.C., Neto, A.A.D., Garnica, A.I.C.: Adsorption of nonionic surfactants in sandstones. Colloids Surf. A, Physicochem. Eng. Asp. 293, 1–4 (2007)
Grigg, R.B., Bai, B.: Calcium lignosulfonate adsorption and desorption on Berea sandstone. J. Colloid Interface Sci. 279, 36–45 (2004)
Iglauer, S., Wu, Y., Shuler, P., Tang, Y., Goddard, W.A.: New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J. Pet. Sci. Eng. 71, 23–29 (2010)
Karsa, D.R.: Industrial Applications of Surfactants IV. RSC, Cambridge (1999)
Király, Z., Dékány, I., Klumpp, E., Lewandowski, H., Narres, H.D., Schwuger, M.J.: Selective sorption of phenol and related compounds from aqueous solutions onto graphitized carbon black. Adsorption and flow microcalorimetric studies. Langmuir 12, 423–430 (1996)
Király, Z., Findenegg, G.H.: Calorimetric study of the adsorption of short-chain nonionic surfactants on silica glass and graphite: dimethyldecylamine oxide and octyl monoglucoside. Langmuir 16, 8842–8849 (2000)
Lawson, J.B., Dilgren, R.E., (Shell Development Co.): Adsorption of sodium alkyl aryl sulfonates on sandstone. Soc. Pet. Eng. J. 18, 75–82 (1978)
Menger, F.M., Keiper, J.S.: Gemini surfactants. Angew. Chem. 39, 1906–1920 (2000)
Myers, D.: Surfactant Science and Technology. VCH, New York (1988)
Øren, P.E., Bakke, S.: Reconstruction of Berea sandstone and pore-scale modeling of wettability effects. J. Pet. Sci. Eng. 39, 177–199 (2003)
Páhi, A.B., Király, Z., Mastalir, A., Dudás, J., Puskás, S., Vágó, A.: Thermodynamics of micelle formation of the counterion coupled gemini surfactant bis(4-(2-dodecyl)benzenesulfonate)-Jeffamine salt and its dynamic adsorption on sandstone. J. Phys. Chem. B 112, 15320–15326 (2008)
Rosen, M.J.: Surfactants and Interfacial Phenomena. Wiley, New York (1989)
Rouquerol, J., Partyka, S.: Adsorption of surfactants on rocks: microcalorimetric approach applied to tertiary oil recovery. J. Chem. Technol. Biotechnol. 31, 584–592 (1981)
van Os, N.M., Haandrikman, G.: Liquid-flow microcalorimetry of surfactant adsorption onto sandstone. 1. Experimental method and initial results. Langmuir 3, 1051–1056 (1987)
van Os, N.M., Haak, J.R., Rupert, L.A.M.: Physico-chemical Properties of Selected Anionic, Cationic and Nonionic Surfactants, pp. 90–96. Elsevier, Amsterdam (1993)
Weifeng, Lv., Bazin, B., Desheng, M., Liu, Q., Han, D., Wu, K.: Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J. Pet. Sci. Eng. 77, 209–218 (2011)
Zana, R., Xia, J.: Gemini Surfactants: Synthesis, Interfacial and Solution-Phase Behavior, and Applications. Surfactant Science Series, vol. 117. Dekker, New York (2004)
Zhang, R., Somasundaran, P.: Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interface Sci. 123, 213–229 (2006)
Acknowledgements
This work was supported by MOL Hungarian Oil and Gas Plc, E&P, New Technologies and R&D, the Hungarian Scientific Research Fund (OTKA 68152), and The Project named “TÁMOP-4.2.1/B-09/1/KONV-2010-0005—Creating the Center of Excellence at the University of Szeged” supported by the European Union and co-financed by the European Regional Development Fund.

Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benkő, M., Puskás, S. & Király, Z. Application of mass spectrometry for study of the adsorption of multicomponent surfactant mixtures at the solid/solution interface. Adsorption 19, 71–76 (2013). https://doi.org/10.1007/s10450-012-9419-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-012-9419-x