Abstract
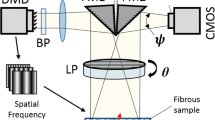
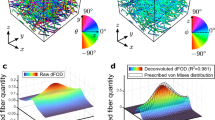
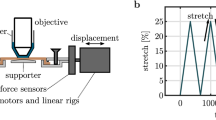
In the present study, we demonstrate that soft tissue fiber architectural maps captured using polarized spatial frequency domain imaging (pSFDI) can be utilized as an effective texture source for DIC-based planar surface strain analyses. Experimental planar biaxial mechanical studies were conducted using pericardium as the exemplar tissue, with simultaneous pSFDI measurements taken. From these measurements, the collagen fiber preferred direction \(\theta _{\text {p}}\) was determined at the pixel level over the entire strain range using established methods (https://doi.org/10.1007/s10439-019-02233-0). We then utilized these pixel-level \(\theta _{\text {p}}\) maps as a texture source to quantify the deformation gradient tensor \({\mathbf {F}}({\mathbf {X}},t)\) as a function of spatial position \({\mathbf {X}}\) within the specimen at time t. Results indicted that that the pSFDI approach produced accurate deformation maps, as validated using both physical markers and conventional particle based method derived from the DIC analysis of the same specimens. We then extended the pSFDI technique to extract the fiber orientation distribution \(\Gamma (\theta ,{\mathbf {X}},t)\) as a function of \({\mathbf {F}}({\mathbf {X}},t)\) from the pSFDI intensity signal. This was accomplished by developing a calibration procedure to account for the optical behavior of the constituent fibers for the soft tissue being studied. We then demonstrated that the extracted \(\Gamma (\theta ,{\mathbf {X}},t)\) was accurately computed in both the referential (i.e. unloaded) and deformed states. Moreover, we noted that the measured \(\Gamma (\theta ,{\mathbf {X}},t)\) agreed well with affine kinematic deformation predictions. We also demonstrated this calibration approach could also be effectively used on electrospun biomaterials, underscoring the general utility of the approach. In a final step, using the ability to simultaneously quantify \({\mathbf {F}}({\mathbf {X}},t)\) and \(\Gamma (\theta ,{\mathbf {X}},t)\), we examined the effect of deformation and collagen structural measurements on the measurement region size. For pericardial tissues, we determined a critical length of \(\sim \) 8 mm wherein the regional variations sufficiently dissipated. This result has immediate potential in the identification of optimal length scales for meso-scale strain measurement in soft tissues and fibrous biomaterials.










Similar content being viewed by others
References
Abramowitz, M., I. A. Stegun, and R. H. Romer. Handbook of mathematical functions with formulas, graphs, and mathematical tables. 56:958–958. ISSN 0002-9505. https://doi.org/10.1119/1.15378.
Agoram, B. and V. H. Barocas. Coupled macroscopic and microscopic scale modeling of fibrillar tissues and tissue equivalents. J. Biomech. Eng., 123(4):362–369, 2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11563762.
Batemen, J.F., S. Lamande, J.A.M. Ramshaw. Collagen superfamily. In: Extracellular Matrix, Volume 2, Molecular Components and Interactions, Volume II, edited by W.D. Comper. Harwood Academic Publishers, Amsterdam, 1996.
Billiar, K. L. and M. S. Sacks. A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J. Biomech. 30(7), 753–756, 1997.
Bodenschatz, N., P. Krauter, A. Liemert, J. Wiest, and A. Kienle. Model-based analysis on the influence of spatial frequency selection in spatial frequency domain imaging. Appl. Opt. 54(22):6725–6731, 2015.
Carleton, J. B., A. D’Amore, K. R. Feaver, G. J. Rodin, and M. S. Sacks. Geometric characterization and simulation of planar layered elastomeric fibrous biomaterials. 12:93–101. ISSN 1878-7568. https://doi.org/10.1016/j.actbio.2014.09.049.
Carleton, J. B. Microscale Modeling of Layered Fibrous Networks with Applications to Biomaterials for Tissue Engineering. PhD thesis, The University of Texas at Austin, 2015.
Carleton, J. B., A. D’Amore, K. R. Feaver, G. J. Rodin, and M. S. Sacks. Geometric characterization and simulation of planar layered elastomeric fibrous biomaterials. Acta Biomater. 12:93–101, 2015. ISSN 1878-7568. https://doi.org/10.1016/j.actbio.2014.09.049.
Carlson, A. B. and P. B. Crilly. Communication systems, 5e, 2010.
Chandran, P. L. and V. H. Barocas. Affine versus non-affine fibril kinematics in collagen networks: theoretical studies of network behavior. J. Biomech. Eng. 128(2):259–270, 2006. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16524339.
Chen, H., Y. Liu, X. Zhao, Y. Lanir, and G. S. Kassab. A Micromechanics Finite-Strain Constitutive Model of Fibrous Tissue. J. Mech. Phys. Solids 59(9):1823–1837, 2011. ISSN 0022-5096 (Electronic) 0022-5096 (Linking). https://doi.org/10.1016/j.jmps.2011.05.012. http://www.ncbi.nlm.nih.gov/pubmed/21927506.
Chesler, N. C. and O. C. Enyinna. Particle deposition in arteries ex vivo: effects of pressure, flow, and waveform. J. Biomech. Eng., 125(3):389–394, 2003. ISSN 0148-0731 (Print) 0148-0731 (Linking). http://www.ncbi.nlm.nih.gov/pubmed/12929244.
Cox, M. A., N. J. Driessen, R. A. Boerboom, C. V. Bouten, and F. P. Baaijens. Mechanical characterization of anisotropic planar biological soft tissues using finite indentation: experimental feasibility. J. Biomech. 41(2):422–429, 2008. ISSN 0021-9290 (Print). https://doi.org/10.1016/j.jbiomech.2007.08.006. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17897653.
Cuccia, D. J., F. Bevilacqua, A. J. Durkin, and B. J. Tromberg. Modulated imaging: quantitative analysis and tomography of turbid media in the spatial-frequency domain. Opt. Lett. 30(11):1354–1356, 2005.
Driessen, N. J. B., R. A. Boerboom, J. M. Huyghe, C. V. Bouten, and F. P. Baaijens. Computational analyses of mechanically induced collagen fiber remodeling in the aortic heart valve. J. Biomech. Eng. 125(4):549–557, 2003. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12968580.
Fan, R. and M. S. Sacks. Simulation of planar soft tissues using a structural constitutive model: Finite element implementation and validation. J. Biomech. 47:2043–2054, 2014.
Fan, R., A. S. Bayoumi, P. Chen, C. M. Hobson, W. R. Wagner, J. E. Mayer, Jr., and M. S. Sacks. Optimal elastomeric scaffold leaflet shape for pulmonary heart valve leaflet replacement. J. Biomech. 46(4):662–669, 2013. ISSN 1873-2380 (Electronic) 0021-9290 (Linking). https://doi.org/10.1016/j.jbiomech.2012.11.046. http://www.ncbi.nlm.nih.gov/pubmed/23294966.
Fung, Y. C. Biomechanics: Motion, Flow, Stress, and Growth. Springer-Verlag, New York, 1990.
Fung, Y. C. Biomechanics: Mechanical Properties of Living Tissues. Springer Verlag, New York, 2nd edition, 1993.
Gilbert, T. W., M. S. Sacks, J. S. Grashow, S. L. Woo, S. F. Badylak, and M. B. Chancellor. Fiber kinematics of small intestinal submucosa under biaxial and uniaxial stretch. J. Biomech. Eng. 128(6):890–898, 2006. ISSN 0148-0731 (Print). http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17154691.
Goldman, H. M., T. G. Bromage, C. D. Thomas, and J. G. Clement. Preferred collagen fiber orientation in the human mid-shaft femur. Anat. Rec. A Discov. Mol. Cell. Evol. Biol., 272(1):434–445, 2003. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12704701.
Goth, W., S. Potter, A. C. B. Allen, J. Zoldan, M. S. Sacks, and J. W. Tunnell. Non-destructive reflectance mapping of collagen fiber alignment in heart valve leaflets. Ann. Biomed. Eng. 47:1250–1264, 2019. ISSN 1573-9686. https://doi.org/10.1007/s10439-019-02233-0.
Goth, W., J. Lesicko, M. S. Sacks, and J. W .Tunnell. Optical-based analysis of soft tissue structures. Annu. Rev. Biomed. Eng. 18:357–385, 2016. ISSN 1545-4274. https://doi.org/10.1146/annurev-bioeng-071114-040625.
Goth, W., B. Yang, J. Lesicko, A. Allen, M.S. Sakcs, and J. W. Tunnel. Polarized spatial frequency domain imaging of heart valve fiber structure. Proceedings of SPIE–--the International Society for Optical Engineering, 2017.
Grashow, J. S., A. P. Yoganathan, and M. S. Sacks. Biaixal stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann. Biomed. Eng. 34(2):315–325, 2006a. ISSN 0090-6964 (Print). https://doi.org/10.1007/s10439-005-9027-y. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16450193.
Grashow, J. S., M. S. Sacks, J. Liao, and A. P. Yoganathan. Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann. Biomed. Eng. 34(10):1509–1518, 2006b. ISSN 0090-6964 (Print). http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17016761.
Guterl, C. C., C. T. Hung, and G. A. Ateshian. Electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage. J. Biomech. 43(7):1343–1350, 2010. ISSN 1873-2380 (Electronic) 0021-9290 (Linking). https://doi.org/10.1016/j.jbiomech.2010.01.021. http://www.ncbi.nlm.nih.gov/pubmed/20189179.
Heo, S. J., N. L. Nerurkar, B. M. Baker, J. W. Shin, D. M. Elliott, and R. L. Mauck. Fiber stretch and reorientation modulates mesenchymal stem cell morphology and fibrous gene expression on oriented nanofibrous microenvironments. Ann. Biomed. Eng. 39(11):2780–2790, 2011. ISSN 1573-9686 (Electronic) 0090-6964 (Linking). https://doi.org/10.1007/s10439-011-0365-7. http://www.ncbi.nlm.nih.gov/pubmed/21800203.
Hu, J. J., G. W. Chen, Y. C. Liu, and S. S. Hsu. Influence of Specimen Geometry on the Estimation of the Planar Biaxial Mechanical Properties of Cruciform Specimens. Exp. Mech. 54(4):615–631, 2014. ISSN 0014-4851. https://doi.org/10.1007/s11340-013-9826-2. http://dx.doi.org/10.1007/s11340-013-9826-2.
Hudson, L. T., S. V. Jett, K. E. Kramer, D. W. Laurence, C. J. Ross, R. A. Towner, R. Baumwart, K. M. Lim, A. Mir, H. M. Burkhart, .Y. Wu, and C.-H. Lee. A pilot study on linking tissue mechanics with load-dependent collagen microstructures in porcine tricuspid valve leaflets. Bioengineering 7(2), 2020. ISSN 2306-5354. https://doi.org/10.3390/bioengineering7020060. https://www.mdpi.com/2306-5354/7/2/60.
Huyghe, J. M. and C. J. Jongeneelen. 3d non-affine finite strains measured in isolated bovine annulus fibrosus tissue samples. Biomech. Model Mechanobiol. 11(1–2):161–170, 2012. ISSN 1617-7940 (Electronic) 1617-7940 (Linking). https://doi.org/10.1007/s10237-011-0300-8. http://www.ncbi.nlm.nih.gov/pubmed/21451947.
Jett, S. V., L. T. Hudson, R. Baumwart, B. N. Bohnstedt, A. Mir, H. M. Burkhart, G. A. Holzapfel, Y. Wu, and C.-H. Lee. Integration of polarized spatial frequency domain imaging (psfdi) with a biaxial mechanical testing system for quantification of load-dependent collagen architecture in soft collagenous tissues. Acta Biomater. 102:149–168, 2020. ISSN 1742-7061. https://doi.org/10.1016/j.actbio.2019.11.028. http://www.sciencedirect.com/science/article/pii/S1742706119307780.
Jor, J. W., P. M. Nielsen, M. P. Nash, and P. J. Hunter. Modelling collagen fibre orientation in porcine skin based upon confocal laser scanning microscopy. Skin Res. Tech. 17(2):149–159, 2011a. ISSN 1600-0846 (Electronic) 0909-752X (Linking). https://doi.org/10.1111/j.1600-0846.2011.00471.x. http://www.ncbi.nlm.nih.gov/pubmed/21241367.
Jor, J. W., M. P. Nash, P. M. Nielsen, and P. J. Hunter. Estimating material parameters of a structurally based constitutive relation for skin mechanics. Biomech. Model. Mechanobiol. 10(5):767–778, 2011b. ISSN 1617-7940 (Electronic) 1617-7940 (Linking). https://doi.org/10.1007/s10237-010-0272-0. http://www.ncbi.nlm.nih.gov/pubmed/21107636.
Jor, J. W., M. P. Nash, P. M. Nielsen, and P. J. Hunter. Modelling the mechanical properties of human skin: towards a 3d discrete fibre model. Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference, 2007:6641–6644, 2007. ISSN 1557-170X (Print) 1557-170X (Linking). https://doi.org/10.1109/IEMBS.2007.4353882. http://www.ncbi.nlm.nih.gov/pubmed/18003548.
Kassab, G. S. and M. S. Sacks, editors. Structure-Based Mechanics of Tissues and Organs. Springer US, 2016. https://doi.org/10.1007/978-1-4899-7630-7.
Konecky, S. D., A. Mazhar, D. Cuccia, A. J. Durkin, J. C. Schotland, and B. J. Tromberg. Quantitative optical tomography of sub-surface heterogeneities using spatially modulated structured light. Opt. Express 17(17):14780–14790, 2009.
Lanir, Y. A Structural Theory for the Homogeneous Biaxial Stress–Strain Relationships in Flat Collageneous Tissues. J. Biomech., 12:423–436, 1979.
Lanir, Y. Plausibility of Structural Constitutive-Equations for Isotropic Soft-Tissues in Finite Static Deformations. J. Appl. Mech. Trans. ASME 61(3):695–702, 1994. ://A1994PJ70400029.
Lanir, Y. Constitutive equations for the lung tissue. J. Biomech. Eng. 105(4):374–380, 1983. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6645447.
Lee, C.-H., W. Zhang, J. Liao, C. A. Carruthers, J. I. Sacks, and M. S. Sacks. On the presence of affine fibril and fiber kinematics in the mitral valve anterior leaflet. Biophys. J. 108(8), 2074–2087, 2015a.
Lee, C.-H., P. J. A. Oomen, J. P. Rabbah, A. Yoganathan, R. C. Gorman, J.H. Gorman, III, R. Amini, and M. S Sacks. A High-Fidelity and Micro-anatomically Accurate 3d Finite Element Model for Simulations of Functional Mitral Valve. In Sébastien Ourselin, Daniel Rueckert, and Nicolas Smith, editors, Functional Imaging and Modeling of the Heart, volume 7945 of Lecture Notes in Computer Science, pages 416–424. Springer Berlin Heidelberg, 2013. ISBN 978-3-642-38898-9. https://doi.org/10.1007/978-3-642-38899-6_49.
Lee, C. H., W. Zhang, J. Liao, C. A. Carruthers, J. I. Sacks, and M. S. Sacks. On the presence of affine fibril and fiber kinematics in the mitral valve anterior leaflet. Biophys. J. 108(8):2074–2087, 2015b. ISSN 1542-0086 (Electronic) 0006-3495 (Linking). https://doi.org/10.1016/j.bpj.2015.03.019. http://www.ncbi.nlm.nih.gov/pubmed/25902446.
Liao, J., L. Yang, J. Grashow, and M.S. Sacks. Collagen fibril kinematics in mitral valve leaflet under biaxial elongation, creep, and stress relaxation. SHVD, 2005.
Neil, M. A. A., R. Juškaitis, and T. Wilson. Method of obtaining optical sectioning by using structured light in a conventional microscope. Opt. Lett. 22(24):1905–1907, 1997.
Olufsen, S. N., M. E. Andersen, and E. Fagerholt. \(\upmu \)dic: an open-source toolkit for digital image correlation. SoftwareX 11:100391, 2020. ISSN 2352-7110. https://doi.org/10.1016/j.softx.2019.100391. http://www.sciencedirect.com/science/article/pii/S2352711019301967.
Ramault, C., A. Makris, D. Van Hemelrijck, E. Lamkanfi, and W. Van Paepegem. Comparison of Different Techniques for Strain Monitoring of a Biaxially Loaded Cruciform Specimen. Strain 47:210–217, 2011. ISSN 1475-1305. https://doi.org/10.1111/j.1475-1305.2010.00760.x. http://dx.doi.org/10.1111/j.1475-1305.2010.00760.x.
Rego, B. V. and M. S. Sacks. A functionally graded material model for the transmural stress distribution of the aortic valve leaflet. J. Biomech. 54:88–95, 2017. ISSN 1873-2380. https://doi.org/10.1016/j.jbiomech.2017.01.039.
Sacks, M. S. and C. J. Chuong. Orthotropic mechanical properties of chemically treated bovine pericardium. Ann. Biomed. Eng. 26(5), 892–902, 1998.
Sacks, M. S. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. Trans. ASME 125:280–287, 2003.
Sacks, M. S. and D. B. Smith. Effects of accelerated testing on porcine bioprosthetic heart valve fiber architecture. Biomaterials, 19(11–12), 1027–1036, 1998.
Sacks, M. S., D. B. Smith, and E. D. Hiester. A small angle light scattering device for planar connective tissue microstructural analysis. Ann. Biomed. Eng. 25(4), 678–689, 1997a.
Sacks, M. S., D. B. Smith, and E. D. Hiester. A small angle light scattering device for planar connective tissue microstructural analysis. Ann. Biomed. Eng. 25(4):678–689, 1997b. ISSN 1573-9686. https://doi.org/10.1007/BF02684845.
Sacks, M. S., D. B. Smith, and E. D. Hiester. The aortic valve microstructure: effects of transvalvular pressure. J. Biomed. Mater. Res. 41(1), 131–141, 1998.
Sacks, M. S. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 125(2):280–287, 2003a. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12751291.
Sacks, M. S., A. Drach, C.-H. Lee, A. H. Khalighi, B.V. Rego, W. Zhang, S. Ayoub, A. P. Yoganathan, R. C. Gorman, and J. H. Gorman. On the simulation of mitral valve function in health, disease, and treatment. J. Biomech. Eng. 141(7), 2019.
Sacks, M. S., Z. He, L. Baijens, S. Wanant, P. Shah, H. Sugimoto, and A. P. Yoganathan. Surface strains in the anterior leaflet of the functioning mitral valve. Ann. Biomed. Eng. 30(10):1281–1290, 2002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12540204.
Sander, E. A., T. Stylianopoulos, R. T. Tranquillo, and V. H. Barocas. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc. Natl. Acad. Sci. USA 106(42):17675–17680, 2009. ISSN 1091-6490 (Electronic) 0027-8424 (Linking). https://doi.org/10.1073/pnas.0903716106. http://www.ncbi.nlm.nih.gov/pubmed/19805118.
Schenke-Layland, K. Non-invasive multiphoton imaging of extracellular matrix structures. J. Biophoton. 1(6):451–462, 2008. ISSN 1864-0648. https://doi.org/10.1002/jbio.200810045.
Shi, Y. and I. Vesely. Morphology of collagen fibers and elastin sheath in tissue-engineered mitral valve chordae. J. Heart Valve Dis..
Stella, J. A., A. D’Amore, W. R. Wagner, and M. S. Sacks. On the biomechanical function of scaffolds for engineering load-bearing soft tissues. Acta Biomater. 6(7), 2365–2381, 2010.
Thornton, G. M., N. G. Shrive, and C. B. Frank. Ligament creep recruits fibres at low stresses and can lead to modulus-reducing fibre damage at higher creep stresses: a study in rabbit medial collateral ligament model. J. Orthop. Res. 20(5):967–974, 2002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12382961.
van Lieshout, M. I., C. M. Vaz, M. C. Rutten, G. W. Peters, and F. P. Baaijens. Electrospinning versus knitting: two scaffolds for tissue engineering of the aortic valve. J. Biomater. Sci. Polym. Ed. 17(1–2):77–89, 2006. ISSN 0920-5063 (Print). http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16411600.
van Putten, S., Y. Shafieyan, and B. Hinz. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 93:133–142, 2016. ISSN 1095-8584 (Electronic) 0022-2828 (Linking). https://doi.org/10.1016/j.yjmcc.2015.11.025. http://www.ncbi.nlm.nih.gov/pubmed/26620422.
Wells, P. B., A. T. Yeh, and J. D. Humphrey. Influence of glycerol on the mechanical reversibility and thermal damage susceptibility of collagenous tissues. IEEE Trans. Biomed. Eng. 53(4):747–753, 2006. ISSN 0018-9294 (Print). http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16602582.
Yang, B., J. Lesicko, M. Sharma, M. Hill, M. S. Sacks, and J. W. Tunnell. Polarized light spatial frequency domain imaging for non-destructive quantification of soft tissue fibrous structures. Biomed. Opt. Express 6(4), 1520–1533, 2015. https://doi.org/10.1364/BOE.6.001520. http://www.osapublishing.org/boe/abstract.cfm?URI=boe-6-4-1520.
Zhang, L., S. P. Lake, V. K. Lai, C. R. Picu, V. H. Barocas, and M. S. Shephard. A coupled fiber-matrix model demonstrates highly inhomogeneous microstructural interactions in soft tissues under tensile load. J. Biomech. Eng. 135(1):011008, 2013a. ISSN 1528-8951 (Electronic) 0148-0731 (Linking). https://doi.org/10.1115/1.4023136. http://www.ncbi.nlm.nih.gov/pubmed/23363219.
Zhang, L., S. P. Lake, V. H. Barocas, M. S. Shephard, and R. C. Picu. Cross-Linked Fiber Network Embedded in Elastic Matrix. Soft Matter 9(28):6398–6405, 2013b. ISSN 1744-683X (Print) 1744-683X (Linking). https://doi.org/10.1039/C3SM50838B. http://www.ncbi.nlm.nih.gov/pubmed/24089623.
Zhang, W., S. Ayoub, J. Liao, and M. S. Sacks. A meso-scale layer-specific structural constitutive model of the mitral heart valve leaflets. Acta Biomater. 32:238–255, 2016. ISSN 1878-7568 (Electronic) 1742-7061 (Linking). https://doi.org/10.1016/j.actbio.2015.12.001. http://www.ncbi.nlm.nih.gov/pubmed/26712602.
Zhang, W., S. Ayoub, J. Liao, and M. S. Sacks. On the mechanical role of collagen and elastin fibers in the layers of the mitral heart valve leaflet. J. Mech. Behav. Biomed. Mater. 2015.
Acknowledgments
The authors would like to acknowledge the Moss Heart Foundation and NIH Grant Nos. R01 HL142504,R01 HL073021, and HL129077. The authors also wish to thank Dr Sindre Nordmark Olufsen of the Norwegian University of Science and Technology for support with the µDIC software package.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Lyndia (Chun) Wu oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Appendix 1: Surface Strain Analysis
From the post-processed imaging data, DIC analysis was carried out using the open-source software \(\mu \)DIC.46 In brief, \(\mu \)DIC takes pixel-level measurements of the gray-scale intensities of a reference image and registers their positions to a finite element surface (Fig. 11). B-splines are used to discretize the deformation field into a bi-directional grid of \({\mathbf {m}} \times {\mathbf {n}}\) control points. The control points are used to manipulate the spline field and the coordinates \({\mathbf {x}}(u,v)\) on the B-spline surface are
where \(N_{i,p}\) and \(N_{j,q}\) are the B-spline basis functions, \(u,v \in [0, 1]\) are the spline coordinates, p, q denote the polynomial order, \({\mathbf {m}}_{ij}\) are the control points’ coordinates. The resulting 2D B-spline surface convects (deforms) and the mapped \(I_{\text {pSFDI}}({\mathbf {X}})\) is compared with the deformed image. A modified Newton scheme was then used to minimize the sum of squared differences between the reference and current images.
This method produced pixel-level resolution spatial maps of \({\mathbf {F}}({\mathbf {X}})\) for each applied deformation step. To allow direct comparison to the physical marker (Fig. 1) \({\mathbf {F}}\) results, the positions of each of the four ROI corners for each loading step (Fig. 11) were extracted and used to compute an equivalent ’virtual’ \({\mathbf {F}}\). Finally, to thoroughly validate pSFDI as a texture source for DIC-based strain measurement, we conducted extensive synthetic deformation validation studies. Reference images of both the pSFDI and particle textures were synthetically deformed in relevant modes. Details of the synthetic testing methods and results can be found in the Appendix.
Appendix 2: Synthetic Deformation Validation
Overview
This section covers the synthetic image testing that was used to confirm that the pSFDI texture source could be utilized to carry-out DIC analysis. Reference images of soft-tissue specimens, one with a physically applied particle texture and another imaged with pSFDI were synthetically deformed in modes that were relevant to the tissue testing performed on the specimens. The synthetic deformations were administered by applying a known F and the effectiveness of the texture for the application of DIC analysis was verified by the successful extraction of synthetic F. The synthetic deformations were both homogeneous (uniaxial stretch, biaxial stretch, simple shear, pure shear, subsimple shear) and heterogeneous(uniaxial and biaxial sinusoidal displacement fields). Rigid body transformations were also applied (translation and rotation).
Software Pipeline
An in-house code was used to generate the synthetic image sets that operates as follows; The type of synthetic deformation is selected, then the number of deformation steps and their individual extent is chosen. Once the synthetic images have been generated, they are passed to the µDIC solver where an ROI and interpolation degree are applied by the user before finally, after DIC analysis, the individual deformation gradient component tensor fields are outputted (for each synthetic deformation step) as a spatial contour map, the displacement field is outputted as a vector plot and the average deformation gradient tensor components at each step are outputted (Fig. 12).
Rigid Body Motion
Rigid body displacement and rotation (Fig. 13) synthetic image sequences were generated and their direction and magnitudes were successfully ascertained by the DIC analysis. Moreover, both textures produce identical results, which is consistent as the same translation was applied in each case.
Stretch and Shear
Next, synthetic stretch and synthetic simple shear images were produced from both the particle texture (Fig. 14) and the pSFDI(\(\uptheta \)p) reference images (Fig. 15). For the homogeneously deformed synthetic images, it can be seen that the measured deformation was well within 0.5 % of the the applied deformation.
Synthetically applied (a) F11 unidirectional stretch, (b) F12 simple shear, (c) F21 simple shear and (d) F22 unidirectional stretch simple shear generated from an image of the particle textured surface. The measured deformation gradient field is appended to the image and show an excellent agreement between the applied deformation and the measured deformation.
Synthetically applied (a) F11 unidirectional stretch, (b) F12 simple shear and (c) F21 simple shear and (d) F22 unidirectional stretch simple shear generated from an image of the pSFDI textured surface. The measured deformation gradient field is appended to the image and show an excellent agreement between the applied and the measured deformations.
Heterogeneous Deformation Modes
Finally, sinusoidal deformation fields were applied to the reference images to test the ability of the DIC software’s tracking of heterogeneity. These were applied in a single direction (Figs. 16 and 17) and bidirectionally (Figs. 18 and 19). It can be seen that in each case, the undulating deformation is followed effectively by µDIC, using both textures.
(a) A plot of the measured F11 deformation gradient component (red dots) co-plotted with the equivalent synthetic data (black line). (b) Measured deformation gradient component fields for a synthetically applied bidirectional sinusoidal deformation of the pSFDI(\(\uptheta \)p) textured reference image.
Rights and permissions
About this article
Cite this article
Dover, C.M., Goth, W., Goodbrake, C. et al. Simultaneous Wide-Field Planar Strain–Fiber Orientation Distribution Measurement Using Polarized Spatial Domain Imaging. Ann Biomed Eng 50, 253–277 (2022). https://doi.org/10.1007/s10439-021-02889-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02889-7