Abstract
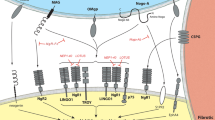
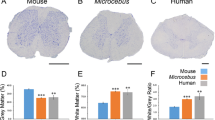
A promising treatment strategy for spinal cord injury (SCI) is to reduce inhibition from chondroitin sulfate proteoglycans (CSPGs). For example, administering intracellular σ peptide (ISP) can improve the ability of axons to cross inhibitory CSPGs and improve function in rodent models of SCI. To translate such treatments into the clinic, we need robust and sensitive methods for studying rodent models. In this study, we applied a newly developed suite of quantitative gait analysis tools: gait analysis instrumentation and technology optimized for rodents (GAITOR), which consists of an arena and open-source software (AGATHA: automated gait analysis through hues and areas). We showed that GAITOR can be used to detect subtle functional improvements (measured by hindlimb duty factor imbalance) in rats following ISP administration in a T10 hemisection injury model. We demonstrated that SCI-specific parameters (right paw placement accuracy and phase dispersion) can be easily added to GAITOR to track recovery. We confirmed the gait observations via retrograde tracer uptake. We concluded that GAITOR is a powerful tool for measuring recovery after moderate/mild SCI, and could be used to replace expensive/inflexible commercially-available gait analysis techniques.





Similar content being viewed by others
References
Basso, D. M., M. S. Beattie, and J. C. Bresnahan. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 12:1–21, 1995.
Burda, J. E. and M. V. Sofroniew. Reactive gliosis and the multicellular response to cns damage and disease. Neuron 81:229–48, 2014.
Ham, T. R. and N. D. Leipzig. Biomaterial strategies for limiting the impact of secondary events following spinal cord injury. Biomed. Mater. 13:024105, 2018.
Jacobs, B. Y., H. E. Kloefkorn, and K. D. Allen. Gait analysis methods for rodent models of osteoarthritis. Curr. Pain. Headache Rep. 18:456, 2014.
Jacobs, B. Y., E. H. Lakes, A. J. Reiter, S. P. Lake, T. R. Ham, N. D. Leipzig, S. L. Porvasnik, C. E. Schmidt, R. A. Wachs, and K. D. Allen. The open source gaitor suite for rodent gait analysis. Sci. Rep. 8:9797, 2018.
Kloefkorn, H. E., B. Y. Jacobs, A. M. Loye, and K. D. Allen. Spatiotemporal gait compensations following medial collateral ligament and medial meniscus injury in the rat: correlating gait patterns to joint damage. Arthritis Res. Ther. 17:287, 2015.
Kloefkorn, H. E., T. R. Pettengill, S. M. Turner, K. A. Streeter, E. J. Gonzalez-Rothi, D. D. Fuller, and K. D. Allen. Automated gait analysis through hues and areas (agatha): a method to characterize the spatiotemporal pattern of rat gait. Ann. Biomed. Eng. 45:711–25, 2016.
Kloos, A. D., L. C. Fisher, M. R. Detloff, D. L. Hassenzahl, and D. M. Basso. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 191:251–65, 2005.
Lang, B. T., J. M. Cregg, M. A. DePaul, A. P. Tran, K. Xu, S. M. Dyck, K. M. Madalena, B. P. Brown, Y.-L. Weng, S. Li, S. Karimi-Abdolrezaee, S. A. Busch, Y. Shen, and J. Silver. Modulation of the proteoglycan receptor ptpsigma promotes recovery after spinal cord injury. Nature 518:404, 2014.
Li, H., T. R. Ham, N. Neill, M. Farrag, A. E. Mohrman, A. M. Koenig, and N. D. Leipzig. A hydrogel bridge incorporating immobilized growth factors and neural stem/progenitor cells to treat spinal cord injury. Adv. Healthc. Mater. 5:802–812, 2016.
Mohan, R., A. P. Tosolini, and R. Morris. Segmental distribution of the motor neuron columns that supply the rat hindlimb: a muscle/motor neuron tract-tracing analysis targeting the motor end plates. Neuroscience 307:98–108, 2015.
Okada, S., M. Hara, K. Kobayakawa, Y. Matsumoto, and Y. Nakashima. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 126:39–43, 2018.
Redondo-Castro, E., A. Torres-Espin, G. Garcia-Alias, and X. Navarro. Quantitative assessment of locomotion and interlimb coordination in rats after different spinal cord injuries. J. Neurosci. Methods 213:165–78, 2013.
Sabelstrom, H., M. Stenudd, and J. Frisen. Neural stem cells in the adult spinal cord. Exp. Neurol. 260:44–9, 2014.
Tsai, E. C., R. L. van Bendegem, S. W. Hwang, and C. H. Tator. A novel method for simultaneous anterograde and retrograde labeling of spinal cord motor tracts in the same animal. J. Histochem. Cytochem. 49:1111–22, 2001.
Acknowledgments
The authors thank Dr. Hossein Tavana (UA) for the use of his Leica Cryostat and the faculty/staff at the Ohio State University Spinal Cord Injury Training Program (sponsored by the Craig H. Neilsen Foundation) for their valuable input and advice. This work was supported by the National Institute of Neurological Disorders and Stroke (Grant 1R21NS096571-01).
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate editor Michael S. Detamore oversaw the review of the article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ham, T.R., Farrag, M., Soltisz, A.M. et al. Automated Gait Analysis Detects Improvements after Intracellular σ Peptide Administration in a Rat Hemisection Model of Spinal Cord Injury. Ann Biomed Eng 47, 744–753 (2019). https://doi.org/10.1007/s10439-019-02198-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02198-0