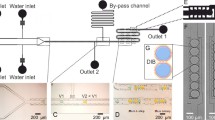
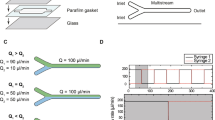
Abstract
There is growing recognition that lipids play key roles in ion channel physiology, both through the dynamic formation and dissolution of lipid ion channels and by indirect regulation of protein ion channels. Because existing technologies cannot rapidly modulate the local (bio)chemical conditions at artificial bilayer lipid membranes used in ion channel studies, the ability to elucidate the dynamics of these lipid–lipid and lipid–protein interactions has been limited. Here we demonstrate a microfluidic system supporting exceptionally rapid perfusion of reagents to an on-chip bilayer lipid membrane, enabling the responses of lipid ion channels to dynamic changes in membrane boundary conditions to be probed. The thermoplastic microfluidic system allows initial perfusion of reagents to the membrane in less than 1 s, and enables kinetic behaviors with time constants below 10 s to be directly measured. Application of the platform is demonstrated toward kinetic studies of ceramide, a biologically important lipid known to self-assemble into transmembrane ion channels, in response to dynamic treatments of small ions (La3+) and proteins (Bcl-xL mutant). The results reveal the broader potential of the technology for studies of membrane biophysics, including lipid ion channel dynamics, lipid–protein interactions, and the regulation of protein ion channels by lipid micro domains.





Similar content being viewed by others
References
Anishkin, A., S. Sukharev, and M. Colombini. Searching for the molecular arrangement of transmembrane ceramide channels. Biophys. J. 90:2414–2426, 2006.
Antonov, V. F., V. V. Petrov, A. A. Molnar, D. A. Predvoditelev, and A. S. Ivanov. Appearance of single-ion channels in unmodified lipid bilayer-membranes at the phase-transition temperature. Nature 283:585–586, 1980.
Antonov, V. F., E. Y. Smirnova, and E. V. Shevchenko. Electric-field increases the phase-transition temperature in the bilayer-membrane of phosphatidic-acid. Chem. Phys. Lipids 52:251–257, 1990.
Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. Mitochondria and cell death—mechanistic aspects and methodological issues. Eur. J. Biochem. 264:687–701, 1999.
Billen, L. P., C. L. Kokoski, J. F. Lovell, B. Leber, and D. W. Andrews. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 6:e147, 2008.
Boheim, G., W. Hanke, and H. Eibl. Lipid phase-transition in planar bilayer-membrane and its effect on carrier-mediated and pore-mediated ion-transport. Proc. Natl Acad. Sci. Biol. 77:3403–3407, 1980.
Chen, C. F., J. Liu, L. P. Hromada, C. W. Tsao, C. C. Chang, and D. L. DeVoe. High-pressure needle interface for thermoplastic microfluidics. Lab Chip 9:50–55, 2009.
Cohen, F. S., M. H. Akabas, J. Zimmerberg, and A. Finkelstein. Parameters affecting the fusion of unilamellar phospholipid vesicles with planar bilayer membranes. J. Cell Biol. 98:1054–1062, 1984.
Cohen, F. S., J. Zimmerberg, and A. Finkelstein. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II. Incorporation of a vesicular membrane marker into the planar membrane. J. Gen. Physiol. 75:251–270, 1980.
Colombini, M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim. Biophys. Acta 1797:1239–1244, 2010.
Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341:233–249, 1999.
Dart, C. Lipid microdomains and the regulation of ion channel function. J. Physiol. 588:3169–3178, 2010.
Epshtein, Y., A. P. Chopra, A. Rosenhouse-Dantsker, G. B. Kowalsky, D. E. Logothetis, and I. Levitan. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc. Natl Acad. Sci. USA 106:8055–8060, 2009.
Fantini, J., and F. J. Barrantes. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim. Biophys. Acta 1788:2345–2361, 2009.
Fertig, N., R. H. Blick, and J. C. Behrends. Whole cell patch clamp recording performed on a planar glass chip. Biophys. J. 82:3056–3062, 2002.
Fertig, N., C. Meyer, R. H. Blick, C. Trautmann, and J. C. Behrends. Microstructured glass chip for ion-channel electrophysiology. Phys. Rev. E 64:040901, 2001.
Funakoshi, K., H. Suzuki, and S. Takeuchi. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal. Chem. 78:8169–8174, 2006.
Ganesan, V., and M. Colombini. Regulation of ceramide channels by Bcl-2 family proteins. FEBS Lett. 584:2128–2134, 2010.
Ganesan, V., M. N. Perera, D. Colombini, D. Datskovskiy, K. Chadha, and M. Colombini. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis 15:553–562, 2010.
Hausmann, G., L. A. O’Reilly, R. van Driel, J. G. Beaumont, A. Strasser, J. M. Adams, and D. C. Huang. Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-x(L). J. Cell Biol. 149:623–634, 2000.
Heimburg, T. Lipid ion channels. Biophys. Chem. 150:2–22, 2010.
Heimburg, T., and A. D. Jackson. The thermodynamics of general anesthesia. Biophys. J. 92:3159–3165, 2007.
Holden, M. A., and H. Bayley. Direct introduction of single protein channels and pores into lipid bilayers. J. Am. Chem. Soc. 127:6502–6503, 2005.
Holden, M. A., L. Jayasinghe, O. Daltrop, A. Mason, and H. Bayley. Direct transfer of membrane proteins from bacteria to planar bilayers for rapid screening by single-channel recording. Nat. Chem. Biol. 2:314–318, 2006.
Hromada, L. P., B. J. Nablo, J. J. Kasianowicz, M. A. Gaitan, and D. L. DeVoe. Single molecule measurements within individual membrane-bound ion channels using a polymer-based bilayer lipid membrane chip. Lab Chip 8:602–608, 2008.
Hsu, Y. T., K. G. Wolter, and R. J. Youle. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl Acad. Sci. USA 94:3668–3672, 1997.
Jeong, S. Y., B. Gaume, Y. J. Lee, Y. T. Hsu, S. W. Ryu, S. H. Yoon, and R. J. Youle. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 23:2146–2155, 2004.
Kasianowicz, J. J., E. Brandin, D. Branton, and D. W. Deamer. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl Acad. Sci. USA 93:13770–13773, 1996.
Kaufmann, K., and I. Silman. Proton-induced ion channels through lipid bilayer-membranes. Naturwissenschaften 70:147–149, 1983.
Kaufmann, K., and I. Silman. The induction by protons of ion channels through lipid bilayer-membranes. Biophys. Chem. 18:89–99, 1983.
Kroemer, G., B. Dallaporta, and M. Resche-Rigon. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60:619–642, 1998.
Malmstadt, N., M. A. Nash, R. F. Purnell, and J. J. Schmidt. Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett. 6:1961–1965, 2006.
Narula, J., P. Pandey, E. Arbustini, N. Haider, N. Narula, F. D. Kolodgie, B. Dal Bello, M. J. Semigran, A. Bielsa-Masdeu, G. W. Dec, S. Israels, M. Ballester, R. Virmani, S. Saxena, and S. Kharbanda. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl Acad. Sci. USA 96:8144–8149, 1999.
Papahadj, D., K. Jacobson, S. Nir, and T. Isac. Phase-transitions in phospholipid vesicles—fluorescence polarization and permeability measurements concerning effect of temperature and cholesterol. Biochim. Biophys. Acta 311:330–348, 1973.
Patel, H. H., F. Murray, and P. A. Insel. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. 48:359–391, 2008.
Sabra, M. C., K. Jorgensen, and O. G. Mouritsen. Lindane suppresses the lipid-bilayer permeability in the main transition region. Biochim. Biophys. Acta 1282:85–92, 1996.
Sandison, M. E., and H. Morgan. Rapid fabrication of polymer microfluidic systems for the production of artificial lipid bilayers. J. Micromech. Microeng. 15:S139–S144, 2005.
Sandison, M. E., M. Zagnoni, M. Abu-Hantash, and H. Morgan. Micromachined glass apertures for artificial lipid bilayer formation in a microfluidic system. J. Micromech. Microeng. 17:S189–S196, 2007.
Sandison, M. E., M. Zagnoni, and H. Morgan. Air-exposure technique for the formation of artificial lipid bilayers in microsystems. Langmuir 23:8277–8284, 2007.
Schindler, H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 122:77–79, 1980.
Simons, K., and E. Ikonen. Functional rafts in cell membranes. Nature 387:569–572, 1997.
Simons, K., and D. Toomre. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39, 2000.
Siskind, L. J., and M. Colombini. The lipids C-2- and C-16-ceramide form large stable channels—implications for apoptosis. J. Biol. Chem. 275:38640–38644, 2000.
Siskind, L. J., A. Davoody, N. Lewin, S. Marshall, and M. Colombini. Enlargement and contracture of C-2-ceramide channels. Biophys. J. 85:1560–1575, 2003.
Siskind, L. J., L. Feinstein, T. X. Yu, J. S. Davis, D. Jones, J. Choi, J. E. Zuckerman, W. Z. Tan, R. B. Hill, J. M. Hardwick, and M. Colombini. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J. Biol. Chem. 283:6622–6630, 2008.
Siskind, L. J., S. Fluss, M. Bui, and M. Colombini. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J. Bioenerg. Biomembr. 37:227–236, 2005.
Siskind, L. J., R. N. Kolesnick, and M. Colombini. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277:26796–26803, 2002.
Siskind, L. J., R. N. Kolesnick, and M. Colombini. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6:118–125, 2006.
Susin, S. A., N. Zamzami, and G. Kroemer. Mitochondria as regulators of apoptosis: doubt no more. Biochim. Biophys. Acta 1366:151–165, 1998.
Suzuki, H., K. Tabata, Y. Kato-Yamada, H. Noji, and S. Takeuchi. Planar lipid bilayer reconstitution with a micro-fluidic system. Lab Chip 4:502–505, 2004.
Suzuki, H., K. V. Tabata, H. Noji, and S. Takeuchi. Highly reproducible method of planar lipid bilayer reconstitution in polymethyl methacrylate microfluidic chip. Langmuir 22:1937–1942, 2006.
Taylor, G. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. R. Soc. Lond. A Math. Phys. Sci. 219:8, 1953.
Trauble, H., M. Teubner, P. Woolley, and H. Eibl. Electrostatic interactions at charged lipid-membranes. 1. Effects of pH and univalent cations on membrane structure. Biophys. Chem. 4:319–342, 1976.
Urbankova, E., A. Voltchenko, P. Pohl, P. Jezek, and E. E. Pohl. Transport kinetics of uncoupling proteins. Analysis of UCP1 reconstituted in planar lipid bilayers. J. Biol. Chem. 278:32497–32500, 2003.
Wiesner, D. A., J. P. Kilkus, A. R. Gottschalk, J. Quintans, and G. Dawson. Anti-immunoglobulin-induced apoptosis in WEHI 231 cells involves the slow formation of ceramide from sphingomyelin and is blocked by bcl-XL. J. Biol. Chem. 272:9868–9876, 1997.
Woodbury, D. J. Nystatin/ergosterol method for reconstituting ion channels into planar lipid bilayers. Meth. Enzymol. 294:319–339, 1999.
Woodbury, D. J., and C. Miller. Nystatin-induced liposome fusion. A versatile approach to ion channel reconstitution into planar bilayers. Biophys. J. 58:833–839, 1990.
Woodbury, D. J., and K. Rognlien. The t-SNARE syntaxin is sufficient for spontaneous fusion of synaptic vesicles to planar membranes. Cell Biol. Int. 24:809–818, 2000.
Yafuso, M., S. J. Kennedy, and A. R. Freeman. Spontaneous conductance changes, multilevel conductance states and negative differential resistance in oxidized cholesterol black lipid-membranes. J. Membr. Biol. 17:201–212, 1974.
Yethon, J. A., R. F. Epand, B. Leber, R. M. Epand, and D. W. Andrews. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 278:48935–48941, 2003.
Zagnoni, M., M. E. Sandison, P. Marius, A. G. Lee, and H. Morgan. Controlled delivery of proteins into bilayer lipid membranes on chip. Lab Chip 7:1176–1183, 2007.
Acknowledgments
The authors gratefully acknowledge Dr. Louis Hromada for input on chip fabrication, Meenu Perera for assistance with pClamp software and Vidyaramanan Ganesan for providing the Bcl-xL mutant. We thank Dr. David W. Andrews at McMaster University in Canada for providing plasmid for protein preparation. This work is supported by NIH grants R01GM072512 and R21EB011750.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Tingrui Pan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Shao, C., Sun, B., Colombini, M. et al. Rapid Microfluidic Perfusion Enabling Kinetic Studies of Lipid Ion Channels in a Bilayer Lipid Membrane Chip. Ann Biomed Eng 39, 2242–2251 (2011). https://doi.org/10.1007/s10439-011-0323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0323-4