Abstract
Background
Standard chemoradiotherapy (CRT) using 5-FU and CDDP is the optimal treatment for patients with stage II/III (non-T4) esophageal carcinoma. However, patient quality of life (QOL) cannot necessarily be maintained during this therapy, because 5-FU must be continuously infused for 24 h and CDDP administration requires a large transfusion volume. Therefore, hospitalization is unavoidable. We conducted a study of definitive CRT with S-1 and nedaplatin.
Methods
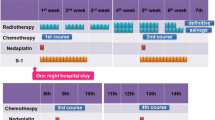
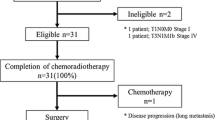
The study was conducted between July 2004 and December 2006. Eligibility criteria were stage II/III (non-T4), PS 0–2, age 20–79 years, and adequate organ function. S-1 80 mg/m2 was given on days 1–14, and nedaplatin 90 mg/m2 on day 1 every 4 weeks. Patients received two courses with concurrent radiotherapy of more than 50 Gy.
Results
Twenty patients (age range, 50–75 years; PS 0/1, 8/12; stage IIA/IIB/III, 11/2/7) were enrolled. Grade 4 leukopenia, thrombocytopenia, and anemia occurred in 15%, 10%, and 5% of patients, respectively. Grade 3 nonhematotoxicity included esophagitis in 3 patients (15%) and anorexia in 2 (10%). One patient developed febrile neutropenia; another developed an esophageal fistula. Complete response was achieved in 80%. The 3-year overall survival rate was 58.0%. Thirteen subjects received treatment as outpatients.
Conclusions
S-1 and nedaplatin in combination with radiotherapy is feasible, and toxicity is tolerable. This treatment method has the potential to shorten hospitalization and maintain patient QOL without impairing the efficacy of CRT.

Similar content being viewed by others
References
Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21.
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74.
Minashi K, Muro K, Katoh K, Ohtsu A, Ishikura S, Boku N, et al. A phase II study of chemoradiotherapy in patients (pts) with stage II–III esophageal squamous cell carcinoma (ESCC): Japan Clinical Oncology Group trial (JCOG 9906). In: Gastrointestinal Cancers Symposium. Orlando: American Society of Clinical Oncology: 2008, abstract 114.
Kleinberg L, Gibson MK, Forastiere AA. Chemoradiotherapy for localized esophageal cancer: regimen selection and molecular mechanisms of radiosensitization. Nat Clin Pract Oncol. 2007;4:282–94.
Sasaki Y, Amano T, Morita M, Shinkai T, Eguchi K, Tamura T, et al. Phase I study and pharmacological analysis of cis-diammine (glycolato) platinum (254-S; NSC 375101D) administered by 5-day continuous intravenous infusion. Cancer Res. 1991;51:1472–7.
Uchida N, Takeda Y, Hojo K, Maekawa R, Sugita K, Yoshioka T. Sequence-dependent antitumour efficacy of combination chemotherapy of nedaplatin, a novel platinum complex, with 5-fluorouracil in an in vivo murine tumour model. Eur J Cancer. 1998;34:1796–801.
Kameyama Y, Okazaki N, Nakagawa M, Koshida H, Nakamura M, Gemba M. Nephrotoxicity of a new platinum compound, 254-S, evaluated with rat kidney cortical slices. Toxicol Lett. 1990;52:15–24.
Grunberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27:2705–14.
Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15:955–9.
Taguchi T, Wakui A, Nabeya K, Kurihara M, Isono K, Kakegawa T, et al. A phase II clinical study of s diammine glycolato platinum, 254-S, for gastrointestinal cancers. 254-S Gastrointestinal Cancer Study Group. Jpn J Cancer Chemother. 1992;19:483–8.
Inaba H, Tsuda T, Miyazaki A, Watanabe Y, Nakaya S, Koitabashi Y, et al. Clinical study of the combination of small amount of nedaplatin (CDGP)/5-FU with radiation for the treatment of esophageal cancer. Jpn J Gastroenterol. 2002;99:1191–6.
Sato Y, Takayama T, Sagawa T, Okamoto T, Miyanishi K, Sato T, et al. A phase I/II study of nedaplatin and 5-fluorouracil with concurrent radiotherapy in patients with esophageal cancer. Cancer Chemother Pharmacol. 2006;58:570–6.
Kodaira T, Fuwa N, Kamata M, Furutani K, Tachibana H, Yamazaki T. Single-institute phase I/II trial of alternating chemoradiotherapy with 5-FU and nedaplatin for esophageal carcinoma. Anticancer Res. 2006;26:471–8.
Ishikura S, Ohtsu A, Shirao K, Muro K, Kagami Y, Nihei K, et al. A phase I/II study of nedaplatin and 5-fluorouracil with concurrent radiotherapy in patients with T4 esophageal cancer: Japan Clinical Oncology Group trial (JCOG 9908). Esophagus. 2005;2:133–7.
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase-II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M Terafur-0.4 M Gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–20.
S-1 Cooperative Study Group, Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B. Late phase-II study of S-1 inpatients with advanced head and neck cancer. Jpn J Cancer Chemother. 2001;28:1381–90.
Fukushima M, Sakamoto K, Sakata M, Nakagawa F, Saito H, Sakata Y. Gimeracil, a component of S-1, may the antitumor activity of X-ray irradiation in human cancer xenograft models in vivo. Oncol Rep. 2010;24:1307–13.
Sobin LH, Fleming ID. TNM classification of malignant tumors, fifth edn (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer (Phila). 1997;80:1803–4.
Inaba H, Tsuda T, Miyazaki A, Obara K, Hattori S, Hosikawa Y, et al. S-1/CDGP radiochemotherapy for esophageal cancer. Gastroenterology. 2006;42:329–32.
Tahara M, Ohtsu A, Hironaka S, Boku N, Ishikura S, Miyata Y, et al. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol. 2005;35:316–23.
Igaki H, Kato H, Ando N, Shinoda M, Shimizu H, Nakamura T, et al. A randomized trial of postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus neoadjuvant chemotherapy for clinical Stage II/III squamous cell carcinoma of the thoracic esophagus (JCOG 9907). Proc Am Soc Clin Oncol 2008;26:abstract 4510.
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71.
Cutsem VE, Köhne CH, Hitre E, Zaluski J, Chien CC, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Tsuda T, Inaba H, Miyazaki A, Takano T, Itoh F. Combination of S-1/nedaplatin and radiotherapy in esophageal cancer: immunohistochemical evaluation of thymidylate synthase, dihydropyrimidine dehydrogenase and p53. St Marianna Med J. 2005;33:515–28.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78.
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–654.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuda, T., Inaba, H., Miyazaki, A. et al. Prospective study of definitive chemoradiotherapy with S-1 and nedaplatin in patients with stage II/III (non-T4) esophageal cancer. Esophagus 8, 45–51 (2011). https://doi.org/10.1007/s10388-011-0261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-011-0261-0