Abstract
Purpose
To investigate longitudinal changes in intraocular pressure (IOP), axial length (AL), and choroidal thickness (ChT) in primary open-angle glaucoma (POAG) eyes after trabeculectomy and to evaluate the parameters that might influence those changes.
Methods
In this prospective observational study, we recruited 28 patients with POAG (28 eyes) scheduled for trabeculectomy. The average macular ChTs and foveal retinal thicknesses along 6-mm segments centered on the fovea were examined preoperatively and postoperatively (at 1, 3, and 6 months) using swept-source optical coherence tomography. The IOP, AL, and mean deviation (MD) of standard automated perimetry (SAP) were also analyzed as independent variables.
Results
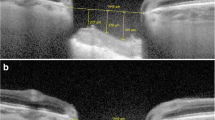
Results from 16 patients were included in the final analysis. A significant increase in ChT with respect to the preoperative value was observed at every postoperative stage (1 month, P < 0.001; 3 months, P < 0.001; 6 months, P = 0.011), whereas the retinal thickness showed no significant change over the study period. The ChT increase and IOP reduction were sustained throughout the 6-month period without further significant changes. Stepwise multivariate analyses showed significant correlations between the percentage decrease in IOP and the percentage increase in ChT at 1 and 6 months postoperatively. The percentage increase in ChT was also significantly correlated with a better MD of the SAP at 1 month (β = 0.01; P = 0.009).
Conclusions
The ChT increase following trabeculectomy was sustained at 1, 3, and 6 months postoperatively. The percentage increase in ChT was significantly correlated with the percentage change in IOP and (more weakly) with better SAP MD values.


Similar content being viewed by others
References
Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40.
Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK. Visual field progression in the Collaborative Initial Glaucoma Treatment Study: the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–7.
Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003;110:1185–91.
Rulli E, Biagioli E, Riva I, Gambirasio G, De Simone I, Floriani I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131:1573–82.
Stamper RL, McMenemy MG, Lieberman MF. Hypotonous maculopathy after trabeculectomy with subconjunctival 5-fluorouracil. Am J Ophthalmol. 1992;114:544–53.
Reis ASC, O’Leary N, Stanfield MJ, Shuba LM, Nicolela MT, Chauhan BC. Laminar displacement and prelaminar tissue thickness change after glaucoma surgery imaged with optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:5819–26.
Yoshikawa M, Akagi T, Hangai M, Ohashi-Ikeda H, Takayama K, Morooka S, et al. Alterations in the neural and connective tissue components of glaucomatous cupping after glaucoma surgery using swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:477–84.
Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012;119:1359–66.
Usui S, Ikuno Y, Uematsu S, Morimoto Y, Yasuno Y, Otori Y. Changes in axial length and choroidal thickness after intraocular pressure reduction resulting from trabeculectomy. Clin Ophthalmol. 2013;7:1155–61.
Saeedi O, Pillar A, Jefferys J, Arora K, Friedman D, Quigley H. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br J Ophthalmol. 2014;98:976–9.
Kara N, Baz O, Altan C, Satana B, Kurt T, Demirok A. Changes in choroidal thickness, axial length, and ocular perfusion pressure accompanying successful glaucoma filtration surgery. Eye (Lond). 2013;27:940–5.
Agoumi Y, Sharpe GP, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118:52–9.
Hata M, Hirose F, Oishi A, Hirami Y, Kurimoto Y. Changes in choroidal thickness and optical axial length accompanying intraocular pressure increase. Jpn J Ophthalmol. 2012;56:564–8.
Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118(1989–94):e2.
Lopilly-Park HY, Jeon SH, Park CK. Enhanced depth imaging detects lamina cribrosa thickness differences in normal tension glaucoma and primary open-angle glaucoma. Ophthalmology. 2012;119:10–20.
Inoue R, Hangai M, Kotera Y, Nakanishi H, Mori S, Morishita S, et al. Three-dimensional high-speed optical coherence tomography imaging of lamina cribrosa in glaucoma. Ophthalmology. 2009;116:214–22.
Lee EJ, Kim TW, Kim M, Kim H. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015;122:721–9.
Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118:1571–9.
Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–5.
Mwanza JC, Sayyad FE, Budenz DL. Choroidal thickness in unilateral advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53:6695–701.
Rhew JY, Kim YT, Choi KR. Measurement of subfoveal choroidal thickness in normal-tension glaucoma in Korean patients. J Glaucoma. 2014;23:46–9.
Wang W, Zhang X. Choroidal thickness and primary open-angle glaucoma: a cross-sectional study and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55:6007–14.
Zhang Z, Yu M, Wang F, Dai Y, Wu Z. Choroidal thickness and open-angle glaucoma: a meta-analysis and systematic review. J Glaucoma. 2016;25:e446–54.
Jonas JB, Steinmetz P, Forster TM, Schlichtenbrede FC, Harder BC. Choroidal thickness in open-angle glaucoma. J Glaucoma. 2015;24:619–23.
Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011;92:189–94.
Li L, Bian A, Zhou Q, Mao J. Peripapillary choroidal thickness in both eyes of glaucoma patients with unilateral visual field loss. Am J Ophthalmol. 2013;156(1277–84):e1.
Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012;12:29.
Park HY, Lee NY, Shin HY, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014;23:225–31.
Roberts KF, Artes PH, O’Leary N, Reis ASC, Sharpe GP, Hutchison DM, et al. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol. 2012;130:980–6.
Sigler EJ, Mascarenhas KG, Tsai JC, Loewen NA. Clinicopathologic correlation of disc and peripapillary region using SD-OCT. Optom Vis Sci. 2013;90:84–93.
Usui S, Ikuno Y, Miki A, Matsushita K, Yasuno Y, Nishida K. Evaluation of the choroidal thickness using high-penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma. Am J Ophthalmol. 2012;153(10–6):e1.
Yamada H, Akagi T, Nakanishi H, Ikeda HO, Kimura Y, Suda K, et al. Microstructure of peripapillary atrophy and subsequent visual field progression in treated primary open-angle glaucoma. Ophthalmology. 2016;123:542–51.
Kim YW, Lee EJ, Kim TW, Kim M, Kim H. Microstructure of β-zone parapapillary atrophy and rate of retinal nerve fiber layer thinning in primary open-angle glaucoma. Ophthalmology. 2014;121:1341–9.
Lee EJ, Kim TW. Lamina cribrosa reversal after trabeculectomy and the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015;122:2234–42.
Hirata M, Tsujikawa A, Matsumoto A, Hangai M, Ooto S, Yamashiro K, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:4971–8.
Akagi T, Hangai M, Kimura Y, Ikeda HO, Nonaka A, Matsumoto A, et al. Peripapillary scleral deformation and retinal nerve fiber damage in high myopia assessed with swept-source optical coherence tomography. Am J Ophthalmol. 2013;155:927–36.
Fleiss JL. The design and analysis of clinical experiments. Hoboken: Wiley; 1999. doi:10.1002/9781118032923.
Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120:175–80.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68.
Riva CE, Titze P, Hero M, Petrig BL. Effect of acute decreases of perfusion pressure on choroidal blood flow in humans. Invest Ophthalmol Vis Sci. 1997;38:1752–60.
Delaey C, Van de Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–56.
Noda Y, Ogawa A, Toyama T, Ueta T. Long-term increase in subfoveal choroidal thickness after surgery for senile cataracts. Am J Ophthalmol. 2014;158(455–9):e1.
Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93.
Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow—relevance for glaucoma. Exp Eye Res. 2011;93:141–55.
Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93.
Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72.
Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma: a population-based assessment. Arch Ophthalmol. 1995;113:216–21.
Usui S, Ikuno Y, Akiba M, Maruko I, Sekiryu T, Nishida K, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53:2300–7.
Saito M, Kano M, Itagaki K, Ise S, Imaizumi K, Sekiryu T. Subfoveal choroidal thickness in polypoidal choroidal vasculopathy after switching to intravitreal aflibercept injection. Jpn J Ophthalmol. 2016;60:35–41.
Lee TG, Yu SY, Kwak HW. Variations in choroidal thickness after high-dose systemic corticosteroid treatment in children with chronic glomerulonephritis. Retina. 2015;35:2567–73.
Hashimoto Y, Saito W, Saito M, Hirooka K, Mori S, Noda K, et al. Increased choroidal blood flow velocity with regression of unilateral acute idiopathic maculopathy. Jpn J Ophthalmol. 2015;59:252–60.
Acknowledgments
This study received financial support from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT) in the form of Innovative Techno-Hub for Integrated Medical Bio-Imaging of the Project for Developing Innovation Systems, and from the Japan Society for the Promotion of Science (JSPS) (Kakenhi Grant Nos. 24592624 and 25462713).
Conflicts of interest
M. Yoshikawa, None; T. Akagi, Lecture fees (Alcon, Kowa, Pfizer, Santen, Senju); H. Nakanishi, None; H. O. Ikeda, Grants (Banyu Foundation, Japan Intractable Diseases Research Foundation, Japan Research Foundation for Clinical Pharmacology, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Uehara Memorial Foundation, YOKOYAMA Foundation for Clinical Pharmacology); S. Morooka, None; H. Yamada, None; T. Hasegawa, None; Y. Iida, None; N. Yoshimura, Grants (Alcon, Bayer, MSD, Novartis, Ohtsuka, Santen, Senju), Consultant fees (Alcon, Bayer, Canon, Japan Focus, Kowa, MSD, Nidek, Novartis, Ohtsuka, Santen, Senju).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10384_2016_482_MOESM1_ESM.tif
Supplementary Fig. 1 Scatterplots of 3 independent variables: choroidal thickness (ChT), age, and axial length (AL). (a) In our dataset, the older patients had shorter ALs (ρ = -0.745, P < 0.001; Spearman rank correlation). (b) In the univariate analysis, age and ChT were not significantly associated (ρ = -0.019, P = 0.94), probably because of the confounding bias between age and AL. (c) The patients with longer ALs tended to have thinner ChTs (ρ = -0.541, P = 0.030) (TIFF 101 kb)
10384_2016_482_MOESM2_ESM.tif
Supplementary Fig. 2 Scatterplots of the percentage decrease in intraocular pressure (IOP) against the percentage increase in choroidal thickness (ChT) at the postoperative 1-month (a), 3-month (b), and 6-month (c) stages. In the univariate analysis, the percentage increase in ChT was not significantly associated with the percentage decrease in IOP, as shown in Table 4 (TIFF 101 kb)
About this article
Cite this article
Yoshikawa, M., Akagi, T., Nakanishi, H. et al. Longitudinal change in choroidal thickness after trabeculectomy in primary open-angle glaucoma patients. Jpn J Ophthalmol 61, 105–112 (2017). https://doi.org/10.1007/s10384-016-0482-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-016-0482-9