Abstract
Purpose
To evaluate the efficacy and safety of loteprednol etabonate (LE) 0.5 % in vernal keratoconjunctivitis (VKC) patients compared to prednisolone and fluorometholone.
Methods
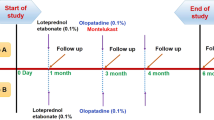
The patients were randomized into three groups: the loteprednol group, prednisolone group and fluorometholone group. Medications were administered four times daily, for a total of 28 days. Before starting treatment and at each visit thereafter, the major symptoms and signs of VKC were recorded and graded as 0 (none), 1 (mild), 2 (moderate) or 3 (severe). Adverse event reports including elevation of intraocular pressure (IOP) were recorded.
Results
There were no significant differences among the groups concerning baseline mean scores of signs and symptoms, which gradually improved in all except for pannus formation in the fluorometholone group. However, all signs and symptoms (except for chemosis) were significantly less improved in the eyes of the fluorometholone group compared with the other groups at each control visit. There was significant IOP elevation after the day 3 visit in the prednisolone group only.
Conclusion
LE was as effective as prednisolone and more effective than fluorometholone, and it had no side effects during the short-term treatment of VKC patients.




Similar content being viewed by others
References
Ehlers WH, Donshik PC. Allergic ocular disorders: a spectrum of diseases. CLAO J. 1992;18:117–24.
Abelson MB, George MA, Garofalo C. Differential diagnosis of ocular allergic disorders. Ann Allergy. 1993;70:95–109.
Shoji J, Inada N, Sawa M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol. 2006;50:195–204.
Kosrirukvongs P, Visitsunthorn N, Vichyanond P, Bunnag C. Allergic conjunctivitis. Asian Pac J Allergy Immunol. 2001;19:237–44.
Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107:1157–63.
Leonardi A, Secchi AG. Vernal keratoconjunctivitis. Int Ophthalmol Clin. 2003;43:41–58.
Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002;21:319–39.
Verin PH, Dicker ID, Mortemousque B. Nedocromil sodium eye drops are more effective than sodium cromoglycate eye drops for the long-term management of vernal keratoconjunctivitis. Clin Exp Allergy. 1999;29:529–36.
BenEzra D. Current practice: diagnosis and treatment in primary healthcare. Allergy. 1995;50:30–3.
Trocme SD, Raizman MB, Bartley GB. Medical therapy for ocular allergy. Mayo Clin Proc. 1995;67:6–9.
Jerndal T, Munkby M. Corticosteroid response in dominant congenital glaucoma. Acta Ophthalmol (Copenh). 1978;56:373–83.
Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–57.
Dell SJ, Lowry GM, Northcutt JA, Howes J, Novack GD, Hart K. A randomized, double-masked, placebo-controlled parallel study of 0.2% loteprednol etabonate in patients with seasonal allergic conjunctivitis. J Allergy Clin Immunol. 1998;102:251–5.
Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30:10–3.
Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1. J Cataract Refract Surg. 1998;24:1480–9.
Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group I. Am J Ophthalmol. 1997;123:455–64.
Loteprednol Etabonate US Uveitis Study Group. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Loteprednol Etabonate US Uveitis Study Group. Am J Ophthalmol. 1999;127:537–44.
Kheirkhah A, Zavareh MK, Farzbod F, Mahbod M, Behrouz MJ. Topical 0.005% tacrolimus eye drop for refractory vernal keratoconjunctivitis. Eye (Lond). 2011;25:872–80.
Uchio E, Itoh Y, Kadonosono K. Topical bromfenac sodium for long-term management of vernal keratoconjunctivitis. Ophthalmologica. 2007;221:153–8.
Bodor N, Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. AAPS J. 2005;7:820–33.
Howes J, Novack GD. Failure to detect systemic levels, and effects of loteprednol etabonate and its metabolite, pj-91, following chronic ocular administration. J Ocul Pharmacol Ther. 1998;14:153–8.
Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–67.
Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9:157–65.
Novack GD, Howes J, Crockett RS, Sherwood MB. Change in intraocular pressure during long-term use of loteprednol etabonate. J Glaucoma. 1998;7:266–9.
Holland EJ, Bartlett JD, Paterno MR, Usner DW, Comstock TL. Effects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteers. Cornea. 2008;27:50–5.
White EM, Macy JI, Bateman KM, Comstock TL. Comparison of the safety and efficacy of loteprednol 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. Curr Med Res Opin. 2008;24:287–96.
Bucala R, Gallati M, Manabe S, Cotlier E, Cerami A. Glucocorticoid-lens protein adducts in experimentally induced steroid cataracts. Exp Eye Res. 1985;40:853–63.
Druzgala P, Wu WM, Bodor N. Ocular absorption and distribution of loteprednol etabonate, a soft steroid, in rabbit eyes. Curr Eye Res. 1991;10:933–7.
Pavesio CE, Decory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol. 2008;92:455–9.
Bilgihan K, Gurelik G, Akata F, Hasanreisoglu B. Fluorometholone induced cataract after photorefractive keratectomy. Ophthalmologica. 1997;211:394–6.
Costagliola C, Cati-Giovannelli B, Priccirrillo A, Delfino M. Cataracts associated with long-term topical steroids. Br J Dermatol. 1989;120:472–3.
Keklikci U, Soker SI, Sakalar YB, Unlu K, Ozekinci S, Tunik S. Efficacy of topical cyclosporin A 0.05% in conjunctival impression cytology specimens and clinical findings of severe vernal keratoconjunctivitis in children. Jpn J Ophthalmol. 2008;52:357–62.
Lambiase A, Leonardi A, Sacchetti M, Deligianni V, Sposato S, Bonini S. Topical cyclosporine prevents seasonal recurrences of vernal keratoconjunctivitis in a randomized, double-masked, controlled 2-year study. J Allergy Clin Immunol. 2011;128:896–7.
Pucci N, Novembre E, Cianferoni A, Lombardi E, Bernardini R, Caputo R, et al. Effi cacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89:298–303.
Vichyanond P, Tantimongkolsuk C, Dumrongkigchaiporn P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P. Vernal keratoconjunctivitis: result of a novel therapy with 0.1% topical ophthalmic FK-506 ointment. J Allergy Clin Immunol. 2004;113:355–8.
Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139:1797–803.
Friedlaender MH. Ocular allergy. Curr Opin Allergy Clin Immunol. 2011;11:477–82.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Öner, V., Türkcü, F.M., Taş, M. et al. Topical loteprednol etabonate 0.5 % for treatment of vernal keratoconjunctivitis: efficacy and safety. Jpn J Ophthalmol 56, 312–318 (2012). https://doi.org/10.1007/s10384-012-0152-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-012-0152-5