Abstract
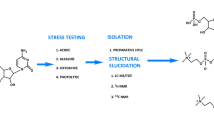
A highly sensitive and simple high-performance liquid chromatographic–tandem mass spectrometric (LC–MS–MS) assay was developed and validated for the quantification of N-acetyl tryptophan (NAT) in formulations and to identify impurities under different stress conditions. N-acetyl tryptophan was analyzed using a reversed-phase gradient elution after treatment under acidic, basic, oxidative, hydrolytic and thermal stress conditions. Linearity in the calibration curve was obtained at a concentration range of 10–100 µgmL−1 (R2 = 0.9916). The lower limits of detection and quantification were 3.53 and 10.69 µgmL−1. The degradation of NAT was observed maximum under oxidation stress (52.84%) and minimum under thermal stress (10.22%). Three major degradation products were formed under acidic and basic stress conditions, of which tryptophan was the major one. Thermal stress yielded a single major impurity at m/z 230. Water hydrolysis could form dihydroxy-N acetyl tryptophan at m/z 279. Oxidation stress led to the formation of seven major degradation products. The most effective stress condition was found to be oxidative which leads to 52.84% degradation of the drug followed by acidic stress (34.64%) and basic stress (15.66%). The present study showed an accurate, precise and sensitive LC–MS–MS method for the systematic investigation of NAT and its impurities in formulations.
Graphic Abstract








Similar content being viewed by others
Abbreviations
- MRM:
-
Multiple Reaction Monitoring
- DiOia:
-
Dioxyindolylalanine
- HSA:
-
Human serum albumin
- ICH:
-
International Conference on Harmonization
- Kyn:
-
Kynurenine
- NAT:
-
N-acetyl-tryptophan
- NFK:
-
N-formyl-kynurenine
- Oia:
-
Oxyindolylalanine
- PIC:
-
H,1,2,3,3a,8,8a-hexahydro-3a-hydroxypyrrolo[2,3-b]indole2-carboxylic acid
- RP:
-
Reversed phase
- Trp:
-
Tryptophan
- AUC:
-
Area under curve
References
Yu MW, Finlayson JS (1984) Quantitative determination of the stabilizers octanoic acid and N-acetyl-DL-tryptophan in human albumin products. J Pharm Sci 73:82–86. https://doi.org/10.1002/jps.2600730122
Zhu T, Chen Z, Zhan C-Y, Wang H-J, Mao L, Lian H-Z (2015) Determination of acetyl-tryptophan in human albumin by reversed-phase high performance liquid chromatography. Asian J Chem. https://doi.org/10.14233/ajchem.2015.17651
Fang L, Parti R, Hu P (2011) Characterization of N-acetyltryptophan degradation products in concentrated human serum albumin solutions and development of an automated high performance liquid chromatography-mass spectrometry method for their quantitation. J Chromatogr A 1218:7316–7324. https://doi.org/10.1016/j.chroma.2011.08.044
Hogan KL, Leiske D, Salisbury CM (2017) Characterization of N-acetyl-tryptophan degradation in protein therapeutic formulations. J Pharm Sci 106:3499–3506. https://doi.org/10.1016/j.xphs.2017.08.012
Nelis HJ, Lefevere MF, Baert E, D’Hoore W, De Leenheer AP (1985) Chromatographic determination of N-acetyl-DL-tryptophan and octanoic acid in human albumin solutions. J Chromatogr 333:381–387
Shrivastava A, Gupta VB (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2:21
Lalitha Devi M, Chandrasekhar KB (2009) A validated stability-indicating RP-HPLC method for levofloxacin in the presence of degradation products, its process related impurities and identification of oxidative degradant. J Pharm Biomed Anal 50:710–717. https://doi.org/10.1016/j.jpba.2009.05.038
Thompson M, Ellison SL, Wood R (2002) Harmonized guidelines for single laboratory validation of methods of analysis. Pure Appl Chem 74:835–855
Kruve A, Rebane R, Kipper K, Oldekop ML, Evard H, Herodes K, Ravio P, Leito I (2015) Tutorial review on validation of liquid chromatography-mass spectrometry methods: part I. Anal Chim Acta 870:29–44. https://doi.org/10.1016/j.aca.2015.02.017
US Food and Drug Administration (2018) Bioanalytical method validation, guidance for industry, US Department of Health and Human Services Food and Drug Administration, Rockville, MD, pp 1–41. https://www.fda.gov/media/70858/download
Nebsen M, Elzanfaly ES (2016) Stability-indicating method and LC–MS–MS characterization of forced degradation products of sofosbuvir. J Chromatogr Sci 54:1–10. https://doi.org/10.1093/chromsci/bmw119
Rao RN, Ramachandra B, Vali RM, Raju SS (2010) LC–MS/MS studies of ritonavir and its forced degradation products. J Pharm Biomed Anal 53:833–842. https://doi.org/10.1016/j.jpba.2010.06.004
ICH (2003) ICH harmonised tripartite guideline: stability testing of new drug substances and products, Q1A (R2) current step 4 version. Geneva, pp 1–24. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2_Guideline.pdf
Simat TJ, Steinhart H (1998) Oxidation of free tryptophan and tryptophan residues in peptides and proteins. J Agric Food Chem 46:490–498. https://doi.org/10.1021/jf970818c
Acknowledgements
The authors would like to thank Institute of Nuclear Medicine and Allied Sciences (INMAS), DRDO, New Delhi for providing all the necessary facilities and requirement to complete this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. There are no conflicts of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agrawal, V., Baghel, R., Singh, A.K. et al. Development and Validation of LC–MS Method for the Estimation of N-Acetyl-Tryptophan and its Impurities Under Stress Conditions. Chromatographia 82, 1467–1477 (2019). https://doi.org/10.1007/s10337-019-03776-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03776-z