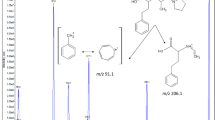
Abstract
A sensitive, high-throughput and economic liquid chromatographic method for determination of fenofibric acid in human plasma was developed and validated by ultraviolet detection and tandem mass spectrometry, then applied in pharmacokinetic study to investigate Lipanthyl™ 200 mg MC bioavailability under food and fasting conditions. Fenofibric acid with 2-chloro fenofibric acid-d6 (internal standard) was extracted from 100 µL of human plasma by acetonitrile in a single extraction step. 25 and 2 µL from supernatant were injected onto ACE column, 50 mm, 5 micron with 4.6 mm inner diameter for LC–UV and 2.1 mm for LC–MS/MS, and both systems were eluted isocratically by water:methanol:formic acid (35:65:0.1, v/v/v), with a constant flow rate of 1 mL min−1. The established calibration curve was linear between 0.05–20 µg mL−1, and the within- and between-day precisions were all below 13 % in both LC–MS/MS and LC–UV systems during validation, and accuracies ranged between 91 and 112 %. Twenty-eight healthy adult subjects participated in this clinical study, and the pharmacokinetic parameters including coefficient of variation were calculated and discussed. A dramatic decrease in C max and AUC0-72 (3.63- and 1.85-fold, respectively) were observed for Lipanthyl™ MC under fasting conditions with more variable inter subject measurements comparing to the fed state.



Similar content being viewed by others
References
Monograph P (2006) Product monograph. Distribution 1–40
Staels B, Dallongeville J, Auwerx J et al (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98:2088–2093
Ramachandran S, Abbas A, Saraf S et al (2012) Significant increase in high-density lipoprotein cholesterol with fibrates is associated with low pretreatment high-density lipoprotein cholesterol: findings from an outpatient clinic setting. Metab Syndr Relat Disord 10:189–194
Wong RPM, Davis TME (2012) In vitro antimalarial activity and drug interactions of fenofibric acid. Antimicrob Agents Chemother 56:2814–2818
Weil A, Caldwell J, Strolin-Benedetti M (1990) The metabolism and disposition of 14C-fenofibrate in human volunteers. Drug Metab Dispos 18:115–120
Chapman MJ (1987) Pharmacology of fenofibrate. Am J Med 83:21–25. doi:10.1016/0002-9343(87)90867-9
Vecera R, Zacharova A, Orolin J et al (2011) Fenofibrate-induced decrease of expression of CYP2C11 and CYP2C6 in rat. Biopharm Drug Dispos 32:482–487
Miller DB, Spence JD (1998) Clinical pharmacokinetics of fibric acid derivatives (Fibrates). Clin Pharmacokinet 34:155–162
Reddy MS, Fazal SM, Apte SS (2011) Solubility enhancement of fenofibrate, a BCS class II drug, by self emulsifying drug delivery systems. Int Res J Pharm 2:173–177
Back H, Song B, Yun H, Chae JA (2015) Mechanistic multi-compartmental Pharmacokinetic model for food effect of fenofibrate. Absr 3362:24
Ling H, Luoma J, Hilleman D (2013) A review of currently available fenofibrate and fenofibric acid formulations. Cardiol Res 4:47–55
Verbeeck RK, Niet S De, Lebrun S, et al. (2015) The Lidose hard capsule formulation of fenofibrate is suprabioavailable compared to the nanoparticle tablet formulation under high-fat fed conditions. J pharm pharm sci 18:61–67
Van Speybroeck M, Mellaerts R, Mols R et al (2010) Enhanced absorption of the poorly soluble drug fenofibrate by tuning its release rate from ordered mesoporous silica. Eur J Pharm Sci 41:623–630
Miller DB, Spence JD (1998) Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet 34:155–162
Abu A, Yacoub M, Alawi M, et al. (2013) Study the influence of low fat content test meal on cyclobenzaprine bioavailability in human body by using LC/MS/MS. Int J Res Pharm Biomed Sci 4:1187–1196
Cancer DOF (2013) United States, p 1
Guivarc’h PH, Vachon MG, Fordyce D (2004) A new fenofibrate formulation: results of six single-dose, clinical studies of bioavailability under fed and fasting conditions. Clin Ther 26:1456–1469
Yun HY, Eun JL, Soo YC et al (2006) The effects of food on the bioavailability of fenofibrate administered orally in healthy volunteers via sustained-release capsule. Clin Pharmacokinet 45:425–432
Sugihara M, Takeuchi S, Sugita M et al (2015) Analysis of intra- and intersubject variability in oral drug absorption in human bioequivalence studies of 113 generic products. Mol Pharm 12:4405–4413
Yamashita S, Tachiki H (2009) Analysis of risk factors in human bioequivalence study that incur bioinequivalence of oral drug products. Mol Pharm 6:48–59
Van Peer A (2010) Variability and impact on design of bioequivalence studies. Basic Clin Pharmacol Toxicol 106:146–153
Straka RJ, Burkhardt RT, Fisher JE (2007) Determination of fenofibric acid concentrations by HPLC after anion exchange solid-phase extraction from human serum. Ther Drug Monit 29:197–202
Mertens B, Cahay B, Klinkenberg R, Streel B (2008) An automated method for the simultaneous determination of pravastatin, 3-hydroxy isomeric metabolite, pravalactone and fenofibric acid in human plasma by sensitive liquid chromatography combined with diode array and tandem mass spectrometry detection. J Chromatogr A 1189:493–502
Streel B, Hubert P, Ceccato A (2000) Determination of fenofibric acid in human plasma using automated solid-phase extraction coupled to liquid chromatography. J Chromatogr Biomed Sci Appl 742:391–400
Stephen J, Barton JB (2014) A novel method for determination of fenofibric acid in human plasma using HPLC-UV: application to a pharmacokinetic study of new formulations. J Anal Bioanal Tech s12:10–13
Kumar A, Monif T, Khuroo AH et al (2010) Development and validation of a LC–ESI–MS/MS method in human plasma for quantification of fenofibric acid, involving chromatographic resolution of fenofibric acid acyl-β-d-glucuronide. Anal Methods 2:1584
Trivedi RK, Kallem RR, Mullangi R, Srinivas NR (2005) Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by LC–MS/MS with electrospray ionization: assay development, validation and application to a clinical study. J Pharm Biomed Anal 39:661–669
Bhavesh D, Shah S, Shivprakash (2009) Determination of fenofibric acid in human plasma by ultra performance liquid chromatography-electrospray ionization mass spectrometry: application to a bioequivalence study. Biomed Chromatogr 23:922–928
Liu A, Patterson AD, Yang Z et al (2009) Fenofibrate metabolism in the cynomolgus monkey using flight mass spectrometry-based metabolomics. Drug Metab Dispos 37:1157–1163
Sunil KD, Manoj S, Tomar, Anil Kumar P, Arshad K, Simrit R, Tausif M (2010) Rapid, sensitive and validated ultra-performance liquid chromatography/mass spectrometric method for the determination of fenofibric acid and its application to human pharmacokinetic study. E-J Chem 7:25–36. doi:10.1155/2010/726124
EMA (2012) Guideline on bioanalytical method validation. EMA Guidel
Food and Drug Administration (2001) Guidance for industry: bioanalytical method validation. US Dep Heal Hum Serv pp 4–10. http://www.labcompliance.de/documents/FDA/FDA-Others/Laboratory/f-507-bioanalytical-4252fnl.pdf
Acknowledgments
This research work was achieved in Jordan center for pharmaceutical research (Amman, Jordan), and we are grateful to the University of Duisburg-Essen/Faculty of Chemistry, (Essen, Germany) for their research facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Informed consent
The informed consent was signed and obtained from each participant volunteers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arafat, T., Arafat, B., Abu Awwad, A. et al. Determination of Fenofibric Acid in Human Plasma by LC–MS/MS and LC–UV. Chromatographia 79, 685–692 (2016). https://doi.org/10.1007/s10337-016-3080-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3080-6