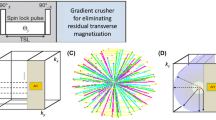
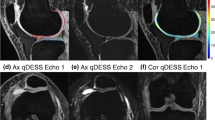
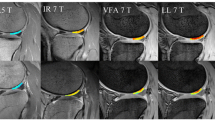
Abstract
Purpose
\(T_2\) mapping is a powerful tool for studying osteoarthritis (OA) changes and bilateral imaging may be useful in investigating the role of between-knee asymmetry in OA onset and progression. The quantitative double-echo in steady-state (qDESS) can provide fast simultaneous bilateral knee \(T_2\) and high-resolution morphometry for cartilage and meniscus. The qDESS uses an analytical signal model to compute \(T_2\) relaxometry maps, which require knowledge of the flip angle (FA). In the presence of \(B_1\) inhomogeneities, inconsistencies between the nominal and actual FA can affect the accuracy of \(T_2\) measurements. We propose a pixel-wise \(B_1\) correction method for qDESS \(T_2\) mapping exploiting an auxiliary \(B_1\) map to compute the actual FA used in the model.
Methods
The technique was validated in a phantom and in vivo with simultaneous bilateral knee imaging. \(T_2\) measurements of femoral cartilage (FC) of both knees of six healthy participants were repeated longitudinally to investigate the association between \(T_2\) variation and \(B_1\).
Results
The results showed that applying the \(B_1\) correction mitigated \(T_2\) variations that were driven by \(B_1\) inhomogeneities. Specifically, \(T_2\) left–right symmetry increased following the \(B_1\) correction (\(\rho _c\) = 0.74 > \(\rho _c\) = 0.69). Without the \(B_1\) correction, \(T_2\) values showed a linear dependence with \(B_1\). The linear coefficient decreased using the \(B_1\) correction (from 24.3 ± 1.6 ms to 4.1 ± 1.8) and the correlation was not statistically significant after the application of the Bonferroni correction (p value > 0.01).
Conclusion
The study showed that \(B_1\) correction could mitigate variations driven by the sensitivity of the qDESS \(T_2\) mapping method to \(B_1\), therefore, increasing the sensitivity to detect real biological changes. The proposed method may improve the robustness of bilateral qDESS \(T_2\) mapping, allowing for an accurate and more efficient evaluation of OA pathways and pathophysiology through longitudinal and cross-sectional studies.







Similar content being viewed by others
Data availability
The MRI data that support the findings of this study are openly available in Zeonodo at https://doi.org/10.5281/zenodo.7478225.
References
Liu F, Chaudhary R, Hurley SA, Munoz Del Rio A, Alexander AL, Samsonov A et al (2014) Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3.0T. J Magn Reson Imaging 39(5):1191–1197. https://doi.org/10.1002/jmri.24290
Ling W, Regatte RR, Navon G, Jerschow A (2008) Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci 105(7):2266–2270. https://doi.org/10.1073/PNAS.0707666105
Monu UD, Jordan CD, Samuelson BL, Hargreaves BA, Gold GE, McWalter EJ (2017) Cluster analysis of quantitative MRI T2 and T1\(\rho\) relaxation times of cartilage identifies differences between healthy and ACL-injured individuals at 3T. Osteoarthr Cartil 25(4):513–520. https://doi.org/10.1016/j.joca.2016.09.015
Jungmann PM, Brucker PU, Baum T, Link TM, Foerschner F, Minzlaff P et al (2015) Bilateral cartilage T2 mapping 9 years after Mega-OATS implantation at the knee: a quantitative 3T MRI study. Osteoarthr Cartil 23(12):2119–2128. https://doi.org/10.1016/j.joca.2015.06.013
Barbieri M, Desai AD, Kogan F, Mazzoli V, Rubin E, Castellani G et al (2019) Rapid Quantitative Bilateral knee imaging with fully automated femoral cartilage analysis. In: Proc. of ISMRM 27th Annual Meeting and Exhibition. Montreal, Canada: Proc. of ISMRM 27th Annual Meeting and Exhibition; 11–16
Black M, Younk K, Chaudhari A, Kogan F, Sveinsson B, McWalter E et al (2020) T2 cluster asymmetry can detect differences in cartilage of ACL-injured subjects 3 months post-surgery. In: Proc. of 2020 International Workshop on Osteoarthritis Imaging. Salzburg, Austria: Proc. of 2020 International Workshop on Osteoarthritis Imaging
Casula V, Tajik BE, Kvist J, Frobell R, Haapea M, Nieminen MT et al (2022) Quantitative evaluation of the tibiofemoral joint cartilage by T2 mapping in patients with acute anterior cruciate ligament injury vs contralateral knees: results from the subacute phase using data from the NACOX study cohort. Osteoarthr Cartil 30(7):987–997. https://doi.org/10.1016/j.joca.2022.02.623
Bruder H, Fischer H, Graumann R, Deimling M (1988) A new steady-state imaging sequence for simultaneous acquisition of two MR images with clearly different contrasts. Magn Reson Med 7(1):35–42. https://doi.org/10.1002/MRM.1910070105
Redpath TW, Jones RA (1988) FADE-A new fast imaging sequence. Magn Reson Med 6(2):224–234. https://doi.org/10.1002/mrm.1910060211
Lee SY, Cho ZH (1988) Fast SSFP gradient echo sequence for simultaneous acquisitions of FID and echo signals. Magn Reson Med 8(2):142–150. https://doi.org/10.1002/mrm.1910080204
Eckstein F, Westhoff J, Sittek H, Maag KP, Haubner M, Faber S et al (2013) In vivo reproducibility of three-dimensional cartilage volume and thickness measurements with MR imaging. Am J Roentgenol 170(3):593–597. https://doi.org/10.2214/AJR.170.3.9490936
Moriya S, Miki Y, Yokobayashi T, Ishikawa M (2009) Three-dimensional double-echo steady-state (3D-DESS) magnetic resonance imaging of the knee: contrast optimization by adjusting flip angle. Acta Radiologica 50(5):507–511. https://doi.org/10.1080/02841850902849444
Hardy PA, Recht MP, Piraino D, Thomasson D (1996) Optimization of a dual echo in the steady state (DESS) free-precession sequence for imaging cartilage. J Magn Reson Imaging 6(2):329–335. https://doi.org/10.1002/JMRI.1880060212
Welsch GH, Scheffler K, Mamisch TC, Hughes T, Millington S, Deimling M et al (2009) Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn Reson Med 62(2):544–549. https://doi.org/10.1002/MRM.22036
Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA (2012) Simultaneous estimation of T2 and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med 67(4):1086–1096. https://doi.org/10.1002/MRM.23090
Sveinsson B, Chaudhari AS, Gold GE, Hargreaves BA (2017) A simple analytic method for estimating T2 in the knee from DESS. Magn Reson Imaging 38:63–70. https://doi.org/10.1016/j.mri.2016.12.018
Chaudhari AS, Black MS, Eijgenraam S, Wirth W, Maschek S, Sveinsson B et al (2018) Five-minute knee MRI for simultaneous morphometry and T2 relaxometry of cartilage and meniscus and for semiquantitative radiological assessment using double-echo in steady-state at 3T. J Magn Reson Imaging 47(5):1328–1341. https://doi.org/10.1002/JMRI.25883
Hennig J (1991) Echoes-how to generate, recognize, use or avoid them in MR-imaging sequences. Part I: fundamental and not so fundamental properties of spin echoes. Concepts Magn Reson 3(3):125–143. https://doi.org/10.1002/cmr.1820030302
Hennig J (1991) Echoes-how to generate, recognize, use or avoid them in MR-imaging sequences. Part II: Echoes in imaging sequences. Concepts Magn Reson 3(4):179–192. https://doi.org/10.1002/cmr.1820030402
Weigel M (2015) Extended phase graphs: dephasing, RF pulses, and echoes—pure and simple. J Magn Reson Imaging 41(2):266–295. https://doi.org/10.1002/jmri.24619
Kogan F, Levine E, Chaudhari AS, Monu UD, Epperson K, Oei EHG et al (2018) Simultaneous bilateral-knee MR imaging. Magn Reson Med 80(2):529–537. https://doi.org/10.1002/MRM.27045
Chaudhari AS, Stevens KJ, Sveinsson B, Wood JP, Beaulieu CF, Oei EHG et al (2019) Combined 5-minute double-echo in steady-state with separated echoes and 2-minute proton-density-weighted 2D FSE sequence for comprehensive whole-joint knee MRI assessment. J Magn Reson Imaging 49(7):e183–e194. https://doi.org/10.1002/jmri.26582
Chaudhari AS, Grissom MJ, Fang Z, Sveinsson B, Lee JH, Gold GE et al (2021) Diagnostic accuracy of quantitative multicontrast 5-minute knee MRI using prospective artificial intelligence image quality enhancement. Am J Roentgenol 216(6):1614–1625. https://doi.org/10.2214/AJR.20.24172. (PMID: 32755384)
Prasloski T, Mädler B, Xiang QS, MacKay A, Jones C (2012) Applications of stimulated echo correction to multicomponent T2 analysis. Magn Reson Med 67(6):1803–1814. https://doi.org/10.1002/MRM.23157
Stollberger R, Wach P (1996) Imaging of the active B1 field in vivo. Magn Reson Med 35(2):246–251. https://doi.org/10.1002/MRM.1910350217
Cunningham CH, Pauly JM, Nayak KS (2006) Saturated double-angle method for rapid B1+ mapping. Magn Reson Med 55(6):1326–1333. https://doi.org/10.1002/MRM.20896
Chung S, Kim D, Breton E, Axel L (2010) Rapid B1+ mapping using a pre-conditioning RF pulse with TurboFLASH readout. Magn Reson Med 64(2):439. https://doi.org/10.1002/MRM.22423
Watkins LE, Rubin EB, Mazzoli V, Uhlrich SD, Desai AD, Black M et al (2020) Rapid volumetric gagCEST imaging of knee articular cartilage at 3 T: evaluation of improved dynamic range and an osteoarthritic population. NMR Biomed 33(8):e4310. https://doi.org/10.1002/nbm.4310
Desai AD, Barbieri M, Mazzoli V, Rubin E, Black M, Watkins L et al (2019) DOSMA: a deep-learning, open-source framework for musculoskeletal MRI analysis. In: Proc. of ISMRM 27th Annual Meeting and Exhibition. Montreal, Canada: Proc. of ISMRM 27th Annual Meeting and Exhibition; 11–16
Desai AD, Gold GE, Hargreaves BA, Chaudhari AS (2019 feb) Technical considerations for semantic segmentation in MRI using Convolutional Neural Networks. arXiv:1902.01977v1 [eessIV]. arXiv:1902.01977
Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW (2010) Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29(1):196–205. https://doi.org/10.1109/TMI.2009.2035616
Shamonin DP, Bron EE, Lelieveldt BPF, Smits M, Klein S, Staring M (2014) Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front Neuroinform 0(JAN):50. https://doi.org/10.3389/FNINF.2013.00050
Jordan CD, McWalter EJ, Monu UD, Watkins RD, Chen W, Bangerter NK et al (2014) Variability of CubeQuant T1\(\rho\), quantitative DESS T2, and cones sodium MRI in knee cartilage. Osteoarthr Cartil 22(10):1559–1567. https://doi.org/10.1016/j.joca.2014.06.001
Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC et al (2011) Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the osteoarthritis initiative. Radiology 261(2):507–515. https://doi.org/10.1148/radiol.11102234
Kretzschmar M, Heilmeier U, Yu A, Joseph GB, Liu F, Solka M et al (2016) Longitudinal analysis of cartilage T2 relaxation times and joint degeneration in African American and Caucasian American women over an observation period of 6 years- Data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 24(8):1384. https://doi.org/10.1016/J.JOCA.2016.03.002
Lansdown DA, Xiao W, Zhang AL, Allen CR, Feeley BT, Li X et al (2020) Quantitative imaging of anterior cruciate ligament (ACL) graft demonstrates longitudinal compositional changes and relationships with clinical outcomes at 2 years after ACL reconstruction. J Orthop Res 38(6):1289–1295. https://doi.org/10.1002/JOR.24572
Williams A, Mikulis B, Krishnan N, Gray M, McKenzie C, Burstein D (2007) Suitability of T1Gd as the “dGEMRIC index’’ at 1.5T and 3.0T. Magn Reson Med 58(4):830–834. https://doi.org/10.1002/MRM.21376
Williams A, Gillis A, McKenzie C, Po B, Sharma L, Micheli L et al (2012) Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Potential Clin Appl 182(1):167–172. https://doi.org/10.2214/AJR.182.1.1820167
Acknowledgements
This work was supported by GE Healthcare and NIH Grants R01-AR077604, R01-EB002524, R01-AR074492, R21EB030180, K24-AR062068, and R00-EB022634.
Author information
Authors and Affiliations
Contributions
MB—study conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision. LEW—study conception and design, acquisition of data and critical revision. VM—study conception and design, and critical revision. ADD—critical revision. ER—acquisition of data. AS— acquisition of the data. GEG—critical revision. BAH—study conception and critical revision. ASC—study conception and critical revision. FK—study conception and design, and critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study received Institutional Review Board approval.
Informed consent
Written informed consent was obtained from the volunteer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barbieri, M., Watkins, L.E., Mazzoli, V. et al. \(B_1\) Field inhomogeneity correction for qDESS \(T_2\) mapping: application to rapid bilateral knee imaging. Magn Reson Mater Phy 36, 711–724 (2023). https://doi.org/10.1007/s10334-023-01094-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-023-01094-y