Abstract
Purpose
The malignancy grades of parotid gland cancer (PGC) have been assessed for a decision of treatment policies. Therefore, we have investigated the feasibility of topology-based radiomic features for the prediction of parotid gland cancer (PGC) malignancy grade in magnetic resonance (MR) images.
Materials and methods
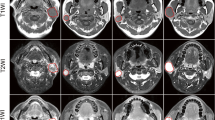
Two-dimensional T1- and T2-weighted MR images of 39 patients with PGC were selected for this study. Imaging properties of PGC can be quantified using the topology, which could be useful for assessing the number of the k-dimensional holes or heterogeneity in PGC regions using invariants of the Betti numbers. Radiomic signatures were constructed from 41,472 features obtained after a harmonization using an elastic net model. PGC patients were stratified using a logistic classification into low/intermediate- and high-grade malignancy groups. The training data were increased by four times to avoid the overfitting problem using a synthetic minority oversampling technique. The proposed approach was assessed using a 4-fold cross-validation test.
Results
The highest accuracy of the proposed approach was 0.975 for the validation cases, whereas that of the conventional approach was 0.694.
Conclusion
This study indicated that topology-based radiomic features could be feasible for the noninvasive prediction of the malignancy grade of PGCs.







Similar content being viewed by others
Data availability
Source codes have been shared in the following URL: https://archive.iii.kyushu-u.ac.jp/public/c9YYQU_INOxYPJOC-84h5gZjD5kHxfVP0Vb1r36O4jlE.
References
Chang JW, Hong HJ, Ban MJ, Shin YS, Kim WS, Koh YW et al (2015) Prognostic factors and treatment outcomes of parotid gland cancer: a 10-year single-center experience. Otolaryngol Head Neck Surg 153(6):981–989
El-Naggar AK, Chan JK, Grandis JR et al (2017) World Health Organization classification of tumours. Chapter 7, tumours of salivary glands. IARC press, Lyon, pp 160–202
Nishikawa S, Kawata R, Higashino M, Lee K, Terada T, Kurisu Y et al (2015) Assessing the histological type and grade of primary parotid carcinoma by fine-needle aspiration and frozen section. Auris Nasus Larynx 42(6):463–468
Omura S, Kawata R, Higashino M, Nishikawa S, Terada T, Haginomori SI, Kurisu Y, Hirose Y (2020) Challenges with preoperative diagnosis of low/intermediate-grade carcinoma of the parotid gland: single-center study of 112 patients. Eur Arch Otorhinolaryngol 277(7):2031–2039. https://doi.org/10.1007/s00405-020-05871-6
Leon B, John WE, Peter R, David S (2005) World Health Organization classification of tumours, pathology and genetics of head and neck tumours, IARC Press, Lyon, pp 209–281
Godballe C, Schultz JH, Krogdahl A, Møller-Grøntved A, Johansen J (2003) Parotid carcinoma: impact of clinical factors on prognosis in a histologically revised series. Laryngoscope 113(8):1411–1417
Lima RA, Tavares MR, Dias FL, Kligerman J, Nascimento MF, Barbosa MM et al (2005) Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol Head Neck Surg 133(5):702–708
Walvekar RR, Filho PAA, Seethala RR, Gooding WE, Heron DE, Johnson JT et al (2011) Clinicopathologic features as stronger prognostic factors than histology or grade in risk stratification of primary parotid malignancies. Head Neck 33(2):225–231
Brierley J, Gospodarowicz MK, Wittekind C (2017) Union for International Cancer Control (UICC) TNM classification of malignant tumours. Wiley Blackwell, Chichester, p 241
Eytan DF, Yin LX, Maleki Z, Koch WM, Tufano RP, Eisele DW et al (2018) Utility of preoperative fine needle aspiration in parotid lesions. Laryngoscope 128(2):398–402
Seethala RR, LiVolsi VA, Baloch ZW (2005) Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck 27(3):217–223
Zbären P, Guélat D, Loosli H, Stauffer E (2008) Parotid tumors: fine-needle aspiration and/or frozen section. Otolaryngol Head Neck Surg 139(6):811–815
Mallon DH, Kostalas M, MacPherson FJ, Parmar A, Drysdale A, Chisholm E et al (2013) The diagnostic value of fine needle aspiration in parotid lumps. Ann R Coll Surg Engl 95(4):258–262
Zhou Z, Qian X, Hu J, Ma X, Zhou S, Dai Y, Zhu J (2021) CT-based peritumoral radiomics signatures for malignancy grading of clear cell renal cell carcinoma. Abdom Radiol 46(6):2690–2698
Gong L, Xu M, Fang M, Zou J, Yang S, Yu X, Tian J (2020) Noninvasive prediction of high-grade prostate cancer via biparametric MRI radiomics. J Magn Reson Imag 52(4):1102–1109
Li S, Liu J, Xiong Y, Pang P, Lei P, Zou H, Luo P (2021) A radiomics approach for automated diagnosis of ovarian neoplasm malignancy in computed tomography. Sci Rep 11(1):1–9
Kamezawa H, Arimura H, Yasumatsu R, Ninomiya K, Haseai S (2020) Preoperative and non-invasive approach for radiomic biomarker-based prediction of malignancy grades in patients with parotid gland cancer in magnetic resonance images. Med Imag Inform Sci 37(4):66–74
Ho K, Lin H, Ann DK, Chu PG, Yen Y (2011) An overview of the rare parotid gland cancer. Head Neck Oncol 3(1):1–7
Lee YYP, Wong KT, King AD, Ahuja AT (2008) Imaging of salivary gland tumours. Eur J Radiol 66(3):419–436
Nakane K, Tsuchihashi Y, Matsuura N (2013) A simple mathematical model utilizing topological invariants for automatic detection of tumor areas in digital tissue images. In Diagnostic Pathology 8(1):1–4. BioMed Central
Ninomiya K, Arimura H (2020) Homological radiomics analysis for prognostic prediction in lung cancer patients. Phys Med 69:90–100
Adcock A, Rubin D, Carlsson G (2014) Classification of hepatic lesions using the matching metric. Comput Vis Image Underst 121:36–42. https://doi.org/10.1016/j.cviu.2013.10.014
Nakane K, Takiyama A, Mori S, Matsuura N (2015) Homology-based method for detecting regions of interest in colonic digital images. Diagn Pathol 10:1–5. https://doi.org/10.1186/s13000-015-0244-x
Le QC, Arimura H, Ninomiya K, Kodama T, Moriyama T (2022) Can persistent homology features capture more intrinsic information about tumors from 18F-fluorodeoxyglucose positron emission tomography/computed tomography images of head and neck cancer patients? Metabolites 12(10):972. https://doi.org/10.3390/metabo12100972
Moons KG, Altman DG, Reitsma JB et al (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162(1):W1-73
Haralick RM, Dinstein I, Shanmugam K (1973) Textural features for image classification. IEEE Trans Syst Man Cybern. https://doi.org/10.1109/TSMC.1973.4309314
Galloway MM (1975) Texture analysis using gray level run lengths. Comput Graph Image Process 4:172–179. https://doi.org/10.1016/S0146-664X(75)80008-6
Thibault G, Fertil B, Navarro C, Pereira S (2009) Texture indexes and gray level size zone matrix application to cell nuclei classification. Pattern Recognit Inf Process pp.140–145
Amadasun M, King R (1989) Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern 19:1264–1274. https://doi.org/10.1109/21.44046
Johnson WE, Li C (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8(1):118–127
Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, Shinohara RT (2017) Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161:149–170
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, Shinohara RT (2018) Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 167:104–120
Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33:1–22. https://doi.org/10.1016/j.expneurol.2008.01.011
Simon N, Friedman J, Hastie T, Tibshirani R (2011) Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw 39:1–13. https://doi.org/10.18637/jss.v039.i05
Sun H, Lin W, Feng R, Li H (2014) Network-regularized high-dimensional Cox regression for analysis of genomic data. Stat Sin 24:1433–1459. https://doi.org/10.5705/ss.2012.317
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49:1373–1379
Arimura H, Soufi M, Ninomiya K, Kamezawa H, Yamada M (2018) Potentials of radiomics for cancer diagnosis and treatment in comparison with computer-aided diagnosis. Radiol Phys Technol 11:365–374
He H, Garcia EA (2009) Learning from Imbalanced Data. IEEE Trans Knowl Data Eng 21(9):1263–1284
Harrel FE (2001) Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer, New York
Ninomiya K, Arimura H, Yoshitake T, Hirose T, Shioyama Y (2022) Synergistic combination of a topologically invariant imaging signature and a biomarker for the accurate prediction of symptomatic radiation pneumonitis before stereotactic ablative radiotherapy for lung cancer: A retrospective analysis. PLoS ONE 17(1):e0263292
Saint Martin MJ, Orlhac F, Akl P, Khalid F, Nioche C, Buvat I, Frouin F (2021) A radiomics pipeline dedicated to Breast MRI: validation on a multi-scanner phantom study. Magn Reson Mater Phy 34(3):355–366
Nemeth A, Chaudet P, Leporq B, Heudel PE, Barabas F, Tredan O, Beuf O (2021) Multicontrast MRI-based radiomics for the prediction of pathological complete response to neoadjuvant chemotherapy in patients with early triple negative breast cancer. Magn Reson Mater Phy 34(6):833–844
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP17K15808. The authors would like to express their gratitude to all members of the Arimura Laboratory (http://www.shs.kyushu-u.ac.jp/~arimura) for their valuable comments for this study.
Author information
Authors and Affiliations
Contributions
Ikushima: study conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision; Arimura: study conception and design, acquisition of data, analysis and interpretation of data, critical revision; Yasumatsu: acquisition of data, analysis and interpretation of data, critical revision; Kamezawa: study conception and design, acquisition of data, analysis and interpretation of data, critical revision; Ninomiya: study conception and design, acquisition of data, analysis and interpretation of data, critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. This study was approved by Institutional Review Board of our hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikushima, K., Arimura, H., Yasumatsu, R. et al. Topology-based radiomic features for prediction of parotid gland cancer malignancy grade in magnetic resonance images. Magn Reson Mater Phy 36, 767–777 (2023). https://doi.org/10.1007/s10334-023-01084-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-023-01084-0