Abstract
Purpose
To develop methods for fluorine-19 (19F) MRI cell tracking in mice on a 3 Tesla clinical scanner. Compared to iron-based cell tracking, 19F MRI has lower sensitivity and, consequently, preclinical 19F cell tracking has only been performed at relatively high magnetic field strengths (> 3 T). Here, we focus on using 19F MRI to detect macrophages in tumors; macrophage density is an indication of tumor aggressiveness and, therefore, 19F MRI could be used as an imaging biomarker.
Methods
Perfluorocarbon (PFC)-labeled macrophages were imaged at 3 T and NMR spectroscopy was performed to validate 19F spin quantification. In vivo 19F MRI was performed on tumor-bearing mice, post-PFC at both 9.4 T and 3 T. 3 T MRI utilized varying NEX and 19F images were analyzed two different ways for 19F quantification.
Results
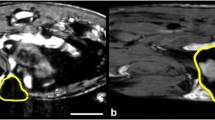
As few as 25,000 cells could be detected as cell pellets at 3 T. 19F quantification in cell pellets by 3 T MRI agreed with NMR spectroscopy. 19F signal was observed in the liver, spleen and tumor in all mice at 9.4 T and 3 T and there was no significant difference in 19F spin quantification.
Conclusion
This study demonstrates the ability to detect and quantify 19F signal in murine tumors using 19F MRI at 3 T.







Similar content being viewed by others
References
Balducci A, Helfer BM, Ahrens ET, O’Hanlon CF 3rd, Wesa AK (2012) Visualizing arthritic inflammation and therapeutic response by fluorine-19 magnetic resonance imaging (19F MRI). J Inflamm (Lond) 9:24
Shin SH, Kadayakkara DK, Bulte JWM (2017) In vivo (19)F MR imaging cell tracking of inflammatory macrophages and site-specific development of colitis-associated dysplasia. Radiology 282:194–201
Zhong J, Narsinh K, Morel PA, Xu H, Ahrens ET (2015) In vivo quantification of inflammation in experimental autoimmune encephalomyelitis rats using fluorine-19 magnetic resonance imaging reveals immune cell recruitment outside the nervous system. PLoS One 10:e0140238
Makela AV, Gaudet JM, Foster PJ (2017) Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Sci Rep 7:42109
Makela AV, Foster PJ (2018) Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magn Reson Med 80:1138–1147
Khurana A, Chapelin F, Xu H, Acevedo JR, Molinolo A, Nguyen Q, Ahrens ET (2018) Visualization of macrophage recruitment in head and neck carcinoma model using fluorine-19 magnetic resonance imaging. Magn Reson Med 79:1972–1980
Shin SH, Park SH, Kang SH, Kim SW, Kim M, Kim D (2017) Fluorine-19 magnetic resonance imaging and positron emission tomography of tumor-associated macrophages and tumor metabolism. Contrast Media Mol Imaging 2017:4896310
Srinivas M, Boehm-Sturm P, Figdor CG, de Vries IJ, Hoehn M (2012) Labeling cells for in vivo tracking using (19)F MRI. Biomaterials 33:8830–8840
Taylor AJ, Granwehr J, Lesbats C, Krupa JL, Six JS, Pavlovskaya GE, Thomas NR, Auer DP, Meersmann T, Faas HM (2016) Probe-specific procedure to estimate sensitivity and detection limits for 19F magnetic resonance imaging. PLoS One 11:e0163704
Temme S, Grapentin C, Quast C, Jacoby C, Grandoch M, Ding Z, Owenier C, Mayenfels F, Fischer JW, Schubert R, Schrader J, Flogel U (2015) Noninvasive imaging of early venous thrombosis by 19F magnetic resonance imaging with targeted perfluorocarbon nanoemulsions. Circulation 131:1405–1414
Ebner B, Behm P, Jacoby C, Burghoff S, French BA, Schrader J, Flogel U (2010) Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ Cardiovasc Imaging 3:202–210
Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C (2011) 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med 65:1144–1153
Flögel U, Su S, Kreideweiß I, Ding Z, Galbarz L, Fu J, Jacoby C, Witzke O, Schrader J (2011) Noninvasive detection of graft rejection by in vivo 19F MRI in the early stage. Am J Transplant 11:235–244
Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET (2007) Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med 58:725–734
Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ (2015) Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One 10:e0118544
Tiainen S, Tumelius R, Rilla K, Hämäläinen K, Tammi M, Tammi R, Kosma VM, Oikari S, Auvinen P (2015) High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 66:873–883
Yuan Z-Y, Luo R-Z, Peng R-J, Wang S-S, Xue C (2014) High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther 7:1475–1480
Gwak JM, Jang MH, Il Kim D, Seo AN, Park SY (2015) Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One 10:1–14
Reigstad I, Smeland HYH, Skogstrand T, Sortland K, Schmid MC, Reed RK, Stuhr L (2016) Stromal integrin α11β1 affects RM11 prostate and 4T1 breast xenograft tumors differently. PLoS One 11:e0151663
Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ, Al-Mehdi A-B (2002) Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol 161:749–753
Gudbjartsson H, Patz S (1995) The rician distribution of noisy MRI data. Magn Reson Med 34:910–914
Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C (2006) In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci USA 103:1852–1857
Zarif L, Postel M, Trevino L, Riess JG, Valla A, Follana R (1994) Biodistribution and excretion of a mixed fluorocarbon-hydrocarbon “dowel” emulsion as determined by 19F NMR. Artif Cells Blood Substit Immobil Biotechnol 22:1193–1198
Ahrens ET, Zhong J (2013) In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 26:860–871
Wang Y-XJ (2011) Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg 1:35–40
Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H (2015) Nanoparticle uptake: the phagocyte problem. Nano Today 10:487–510
Khurana A, Chapelin F, Xu H, Acevedo JR, Molinolo A, Nguyen Q, Ahrens ET (2017) Visualization of macrophage recruitment in head and neck carcinoma model using fluorine-19 magnetic resonance imaging. Magn Reson Med 79:1972–1980
Weibel S, Basse-Luesebrink TC, Hess M, Hofmann E, Seubert C, Langbein-Laugwitz J, Gentschev I, Sturm VJF, Ye Y, Kampf T, Jakob PM, Szalay AA (2013) Imaging of intratumoral inflammation during oncolytic virotherapy of tumors by 19F-magnetic resonance imaging (MRI). PLoS One 8:e56317
Balducci A, Wen Y, Zhang Y, Helfer BM, Hitchens TK, Meng WS, Wesa AK, Janjic JM (2013) A novel probe for the non-invasive detection of tumor-associated inflammation. Oncoimmunology 2:e23034
Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, Coussens LM (2011) MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res 17:5695–5704
Shi Q, Pisani LJ, Lee YK, Messing S, Ansari C, Bhaumik S, Lowery L, Lee BD, Meyer DE, Daldrup-Link HE (2013) Evaluation of the novel USPIO GEH121333 for MR imaging of cancer immune responses. Contrast Media Mol Imaging 8:281–288
Leimgruber A, Berger C, Cortez-Retamozo V, Etzrodt M, Newton AP, Waterman P, Figueiredo JL, Kohler RH, Elpek N, Mempel TR, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ (2009) Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia 11:459-IN4
Ahrens ET, Young W-B, Xu H, Pusateri LK (2011) Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. Biotechniques 50:229–234
Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C (2014) Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med 72:1696–1701
Gocheva V, Wang H-W, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA (2010) IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24:241–255
Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67:2649–2656
Hill HDW, Richards RE (1968) Limits of measurement in magnetic resonance. J Phys E 1:977
Haase A, Odoj F, Von Kienlin M, Warnking J, Fidler F, Weisser A, Nittka M, Rommel E, Lanz T, Kalusche B, Griswold M (2000) NMR probeheads for in vivo applications. Concepts Magn Reson 12:361–388
Morita Y, Zhang R, Leslie M, Adhikari S, Hasan N, Chervoneva I, Rui H, Tanaka T (2017) Pathologic evaluation of tumor-associated macrophage density and vessel inflammation in invasive breast carcinomas. Oncol Lett 14:2111–2118
Acknowledgements
We acknowledge the following sources of funding for AVM: Natural Sciences and Engineering Research Council, Molecular Imaging Graduate Program (Western University), Translational Breast Cancer Research Unit, Cancer Research and Technology Transfer Program and Canadian Cancer Society.
Funding
This study was funded by: Canadian Institute for Health Research.
Author information
Authors and Affiliations
Contributions
AVM study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and critical revision. PJF study conception and design, drafting of manuscript and critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Makela, A.V., Foster, P.J. Preclinical 19F MRI cell tracking at 3 Tesla. Magn Reson Mater Phy 32, 123–132 (2019). https://doi.org/10.1007/s10334-018-0715-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-0715-7