Abstract
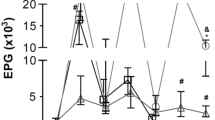
In order to better understand experimental strongyloidiasis in small New World primates, and to evaluate aspects of reinfection and immunosuppression induced by glucocorticoids, nine specimens of Callithrix penicillata (Primates: Cebidae) were administered (by subcutaneous injection, sc) 3000 infective larvae of a strain of Strongyloides venezuelensis (Rhabditida: Strongyloididae) that had been maintained in successive passages through AKR/J mice since 1987. The mean prepatent period was 5.6 ± 0.7 days post-infection (DPI). The mean patent period of infection among the untreated animals (marmosets 1–7) was 123.4 ± 61.4 DPI. Two animals (marmosets 8 and 9) received dexamethasone (2.5 mg/kg, sc) for five consecutive days starting on the 20th day after infection, but this treatment did not alter the course of the infection, and the patent period for these animals was 100.5 ± 58.7 DPI (59 and 142, respectively). Stool examination showed that the highest quantities of parasite eggs were expelled between the 8th and 19th days after inoculation of the larvae. Thereafter, there was a gradual reduction in the number of parasite eggs in feces of all marmosets. During the chronic phase of the infection, before completely negative parasitological findings were obtained, the parasitological examinations were intermittently positive. Reinfection of three of these animals did not result in new positive examinations. However, given the receptiveness of these animals to initial infection with S. venezuelensis and their similarities to human beings, it is proposed that C. penicillata could be used as a nonhuman primate model for experimental strongyloidiasis.



Similar content being viewed by others
References
Amarante AFT, Oliveira-Sequeira TCG (2002) Strongyloides venezuelensis infection susceptibility of seven inbred strains of mice. Arq Bras Med Vet Zootec 54:273–278
Baek BK, Islam MK, Kim BS, Lim CW, Hur J, Oluoch AO, Kim CH, Kakoma I (2003) Characterization of the protective response against a homologous challenge infection with Strongyloides venezuelensis in rats. Vet Parasitol 113:217–227
Baek BK, Whang IS, Islam MK, Kim BS, Kakoma I (2002) Persistent infection with Strongyloides venezuelensis in the Mongolian gerbil (Meriones unguiculatus). Korean J Parasitol 40:181–186
Bailenger J, Carcenac F, Faraggi G, Cabannes A (1976) Rôle de I’hypocorticostéronémie dans le mécanisme de la “self-cure” des rats, parasités par Strongyloides ratti. Ann Parasitol Hum Comp 51:653–665
Barrett KE, Neva FA, Gam AA, Cicmanec J, London WT, Phillips JM, Metcalfe DD (1988) The immune response to nematode parasites: modulation of mast cell numbers and function during Strongyloides stercoralis infections in nonhuman primates. Am J Trop Med Hyg 38:574–581
Brandon DD, Markwick AJ, Chrousos GP, Loriaux DL (1989) Glucocorticoid resistance in humans and nonhumans primates. Cancer Res 49(Suppl 8):2203s–2211s
Denham DA, Suswillo RR, Hetherington CM (1989) Experimental Brugia pahangi and B. malayi infections of callitrichid primates. J Helminthol 63:84–86
Dreyer G, Fernandes-Silva E, Alves S, Rocha A, Albuquerque R, Addiss D (1996) Patterns of detection of Strongyloides stercoralis in stool specimens: implications for diagnosis and clinical trials. J Clin Microbiol 34:2569–2571
Freedman DO (1991) Experimental infection of human subject with Strongyloides species. Rev Infect Dis 13:1221–1226
Gann PH, Neva FA, Gam AA (1994) A randomized trial of single and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. J Infect Dis 169:1076–1079
Gazzinelli SEP (2005) Reinfecção de Mus musculus da linhagem AKR/J por Strongyloides venezuelensis Brumpt, 1934 (dissertation). Universidade Federal de Minas Gerais, Belo Horizonte
Genta RM (1989) Global prevalence of strongyloidiasis: critical review with epidemiologic insight into the prevention of disseminated disease. Rev Infect Dis 11:755–767
Gordon HM, Whitlock HV (1939) A new technique for counting nematode eggs in sheep faeces. J Counc Sci Ind Res 12:50–52
Hiddleston WA (1978) The production of the common marmoset Callithrix penicillata as a laboratory animal. In: Chivers DJ, Lane-Petter W (eds) Recent advances in primatology, vol 2. Academic, London, pp 173–182
Khan AI, Horii Y, Nawa Y (1993a) Defective mucosal immunity and normal systemic immunity of Mongolian gerbils, Meriones unguiculatus, to reinfection with Strongyloides venezuelensis. Parasite Immunol 15:565–571
Khan AI, Horii Y, Tiura R, Sato Y, Nawa Y (1993b) Mucosa mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol 23:551–555
Lunn SF, Hearn JP (1978) Breeding marmosets for medical research. In: Chivers DJ, Lane-Petter W (eds) Recent advances in primatology, vol 2. Academic, London, pp 183–185
Lutz A (1919) O Schistosomum mansoni e a schistosomose, segundo observações feitas no Brasil. Mem Inst Oswaldo Cruz 11:121–155
Martins WA, Nicoli JR, Farias LM, Carvalho MAR, Cara DC, Melo AL (2009) Strongyloides venezuelensis: efeito de antimicrobiano e imunossupressor no curso da infecção em camundongos da linhagem AKR/J. Rev Ciênc Méd Biol 8:315–324
Melo AL (2004) Helminths parasites of Callithrix geoffroyi marmosets (Primates, Callitrichidae). Lab Primate Newsl 43:7–9
Melo AL, Vasconcelos AC, Gazzinelli SEP (2006) Alterações histopatológicas em camundongos AKR/J reinfectados por Strongyloides venezuelensis. Rev Ciênc Méd Biol 5:7–12
Mendes EA, Lima WS, Melo AL (2008) Development of Fasciola hepatica in Lymnaea columella infected with miracidia derived from cattle and marmoset infections. J Helminthol 82:81–84
Moraes RG (1948) Contribuição para o estudo do Strongyloides stercoralis e da estrongiloidíase no Brasil. Rev Serv Espec Saúde Pública 1:507–624
Nakai ES, Amarante AFT (2001) Infecção experimental de camundongos (Mus musculus) e ratos (Rattus norvegicus) com Strongyloides venezuelensis. Rev Bras Parasitol Vet 10:1–6
Nielsen PB, Mojon M (1987) Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zbl Bakt Hyg 263:616–618
Olsen A, Van-Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P (2009) Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103:967–972
Olson CE, Schiller EL (1978) Strongyloides ratti infection in rats. I. Immunopathology. Am J Trop Med Hyg 27:521–526
Orihel TC (1970) The helminth parasites of nonhuman primates and man. Lab Anim Care 20:395–401
Pereira LH, Melo AL (1984) Observações sobre a criação de Callithrix penicillata (Primates, Callitrichidae) em cativeiro. In: Mello MT (ed) A primatologia no Brasil, vol 1. Sociedade Brasileira de Primatologia, Brasília, pp 129–132
Potkay S (1992) Diseases of the Calltrichidae: a review. J Med Primatol 21:189–236
Sato Y, Toma H (1990) Strongyloides venezuelensis infections in mice. Int J Parasitol 20:57–62
Shi BB, Ishikawa N, Khan AI, Tsuchiya K, Horii Y, Nawa Y (1994) Strongyloides venezuelensis infection in Syrian golden hamster, Mesocricetus auratus, with reference to the phenotype of intestinal mucosal mast cells. Parasite Immunol 16:545–551
Tamura N (1993) Studies on availability of Strongyloides venezuelensis for experimental model of strongyloidosis. Bull Nip Vet Anim Sci Univ 59:94–96
Tsuji N, Nakamura Y, Taira N (1993) Long-lasting parasitism of Strongyloides venezuelensis in Mongolian gerbils (Meriones unguiculatus). J Parasitol 79:305–307
Vadlamudi RS, Chi DS, Krishnaswamy G (2006) Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy 4:8
Warren KS, Simões J Jr (1966) The marmoset: a primate resistant to Schistosoma mansoni infection. Am J Trop Med Hyg 15:153–155
Wertheim G (1970) Growth and development of Strongyloides venezuelensis Brumpt, 1934 in the albino rat. Parasitology 61:381–388
Willis HH (1921) A simple levitation method for detection of hookworm ova. Med J Aust 8:375–376
Acknowledgments
To Messrs. Airton Lobo, Bruno Marques Teixeira, and Glaciano Nogueira Ribeiro for technical support, and to CNPq (Conselho Nacional de Pesquisa e Tecnologia) for financial support. The use of marmosets was authorized by the Brazilian Institute of the Environment (Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis, IBAMA), and the experimental procedures were conducted in accordance with the protocol approved by the animal research ethics committee of UFMG.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
de Melo, A.L., Mati, V.L.T. & Martins, W.A. Callithrix penicillata as a nonhuman primate model for strongyloidiasis. Primates 53, 303–309 (2012). https://doi.org/10.1007/s10329-012-0302-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-012-0302-x