Abstract
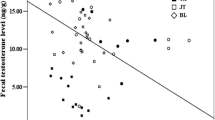
We investigated, longitudinally and cross-sectionally, age and seasonal change in both the testis and nipple volume of Japanese macaques (Macaca fuscata) in relation to concentration profiles of gonadal steroids: testosterone (T) in males and progesterone (P) in females. Testicular volume (TV) and nipple volume (NV) showed rapid growth at puberty, 4.5 and 3.5 years of age in males and females, respectively, but in both sexes there were precocious individuals. The testis as a whole matures at about 10 years of age. TV change is closely related to T concentration profile. The pattern of TV change is composed of maturation and seasonal effects, with individual variation evident mainly in the latter. Some individuals show a simple pattern consisting of one peak in the breeding season (from summer to winter) and one trough in the non-breeding season. Other individuals exhibit a more complicated pattern composed of two or more peaks and troughs before and during the breeding season. The nipple matures at about 7 years but it is difficult to determine the exact maturational age as there are many confounding factors relating to NV. NV shows seasonal fluctuations similar to that of TV. Many animals have periods of substantial growth whereas others do not. The NV in adults from 10 to 25 years does not appear to change much with age, but animals older than 25 years of age have significantly smaller nipples. Seasonal fluctuation in NV mirrors that of the P level. Considered to be controlled by estrogen and P, the NV is a good indicator of the physiological status of reproduction, with its peak about 2 weeks earlier than that of P, that is, at the mid-follicular phase. NV and P level show a similar pattern in pregnancy; from conception, indicated by a P peak, NV and P concentration first decrease, then they increase until peri-parturition and slowly decrease again until the next breeding season.









Similar content being viewed by others
References
Baulieu E-E (1992) Hormones: from molecules to disease. Chapman and Hall, New York
Bielert C (1986) Sexual interactions between captive adult male and female Chacma baboons (Papio ursinus) as related to the female’s menstrual cycle. J Zool (Lond) 209:521–536
Dixson AF (1998) Primate sexuality. Oxford University Press, Oxford
Felig P, Baxter JD, Frohman LA (1995) Endocrinology and metabolism, 3rd edn. McGraw-Hill, New York
Glick BB (1979) Testicular size, testosterone level, and body weight in male Macaca radiata. Folia Primatol 32:268–289
Hamada Y, Hayakawa S, Suzuki J, Ohkura S (1999) Adolescent growth and development in Japanese macaques (Macaca fuscata): punctuated adolescent growth spurt by season. Primates 40:439–452
Hamada Y, Hayakawa S, Suzuki J, Watanabe K, Ohkura S (2003) Body fat and its seasonality in Japanese macaques (Macaca fuscata). Mamm Study 28:79–88
Hamada Y, Iwamoto M, Watanabe T (1986) Somatometrical features of Japanese monkeys in the Koshima Islet: in viewpoint of somatometry, growth, and sexual maturation. Primates 27:471–484
Hamada Y, Watanabe T, Iwamoto M (1996) Physique Index for Japanese macaques (Macaca fuscata): age change and regional variation. Anthropol Sci 104:305–323
Hazama N (1964) Weighing wild Japanese monkeys in Arashiyama. Primates 5(3–4):81–104
Johnson MH, Everitt BJ (1995) Essential reproduction, 4th edn. Blackwell, Oxford
Knobil E, Hotchkiss J (1988) The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill J (eds) The physiology of reproduction, vol 2. Raven, New York, pp 1971–1994
Malina RM, Bouchard C (1991) Growth, maturation, and physical activity. Human Kinetics, Champaign, Ill.
Marson J, Meuris S, Cooper RW, Jouannet P (1991) Puberty in the male chimpanzee: progressive maturation of semen characteristics. Biol Reprod 44:448–455
Mastroianni L, Coutifaris C (1990) Reproductive physiology. In: Rosenfield A, Fathalla MF (eds) The FIGO manual of human reproduction, vol 1. Parthenon, New Jersey
Matsubayashi K, Enomoto T (1983) Longitudinal studies on annual changes in plasma testosterone, body weight and spermatogenesis in adult Japanese monkeys (Macaca fuscata fuscata) under laboratory conditions. Primates 24:521–529
Matsubayashi K, Mochizuki K (1982) Growth of male reproductive organs with observation of their seasonal morphlogic changes in the Japanese monkey (Macaca fuscata). Jpn J Vet Sci 44:891–902
Melnick DJ, Pearl MC (1987) Cercopithecines in multimale groups: genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wranghan RW, Struhsaker TT (eds) Primates societies. University of Chicago Press, Chicago, pp 121–134
Meussy-Dessolle N, Dang DC (1985) Plasma concentration of testosterone, dihydrotestosterone, Δ4-androstenediore, dehydroepiandrosterone and oestradiol-17β in the crab-eating monkeys (Macaca fascicularis) from birth to adulthood. J Reprod Fertil 74:347–359
Mori A, Yamguchi N, Watanabe K, Shimizu K (1997) Sexual maturation of female Japanese macaques under poor nutritional conditions and food-enhanced perineal swelling in the Koshima Troop. Int J Primatol 18:553–579
Nigi H, Morimitsu Y, Hayama S (1995) Correlation between pregnancies and fats accumulated in great omentum in the free-ranging Japanese monkeys (in Japanese). Primate 11:291
Nigi H, Morimitsu Y (1997) Correlation between reproductive success and fats accumulated in greater omemtum in the free-ranging female Japanese monkeys at Takasakiyama (in Japanese). Primate Res 13:236
Nigi H, Tiba T, Yamamoto S, Floescheim Y, Ohsawa N (1980) Sexual maturation and seasonal changes in reproductive phenomena of male Japanese monkeys (Macaca fuscata) at Takasakiyama. Primates 21:230–240
Nozaki M (1991) Mechanisms controlling seasonal breeding in Japanese monkeys (in Japanese with English abstract). Primate Res 7:103–125
Nozaki M (1994) Mechanisms controlling the seasonal breeding of Japanese monkeys (in Japanese with English abstract). J Reprod Dev 40(6):j105–j115
Pavelka MSM, Fedigan LM (1999) Reproductive termination in female Japanese monkeys: a comparative life history perspective. Am J Phys Anthropol 109:455–464
Primate Research Institute (2003) Guide for the care and use of laboratory primates. Primate Research Institute, Kyoto University http://www.pri.kyoto-u.ac.jp/index.html
Rostal DC, Glick BB, Eaton GG, Resko JA (1986) Seasonality of adult male Japanese macaques (Macaca fuscata): androgens and behavior in a confined troop. Horm Behav 20:452–462
Sade DS (1964) Seasonal cycle in size of testes of free-ranging Macaca mulatta. Folia Primatol 2:171–180
Suzuki J, Ohkura S, Hayakawa S, Hamada Y (2000) Time series analysis of plasma insulin-like growth factor-I and gonadal steroids in adolescent Japanese macaques (Macaca fuscata). J Reprod Dev 46:157–166
Takahata Y, Koyama N, Suzuki S (1995) Do the old aged females experience a long post-reproductive life span? The cases of Japanese macaques and chimpanzees. Primates 36:169–180
Tanaka I (1989) Change of nipple preference between successive offspring in Japanese macaques. Am J Primatol 18:321–325
Tanner JM (1962) Growth at adolescence, 2nd edn. Blackwell, Oxford
Terasawa E, Nass TE, Yeoman RR, Loose MD, Schultz NJ (1983) Hypothalamic control of puberty in the female rhesus macaque. In: Norman RL (ed) Neuroendocrine aspects of reproduction: ORPRC symposia on primate reproduction bilogy. Academic, New York, pp 149–182
Vandenbergh JG (1969) Endocrine coordination in monkeys: male sexual responses to the female. Physiol Behav 4:261–264
Acknowledgements
We thank the staff of the section of Morphology and the Center for Human Evolution Modeling Research of the Primate Research Institute, Kyoto University, for their help and valuable suggestions. This research was supported by a grant-in-aid for COE Research 2001, a grant-in-aid for Specially Promoted Research (COE) 2002, and grants-in-aid nos. 11833008, 11304059, and 14204083 from the Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hamada, Y., Suzuki, J., Ohkura, S. et al. Changes in testicular and nipple volume related to age and seasonality in Japanese macaques (Macaca fuscata), especially in the pre- and post-pubertal periods. Primates 46, 33–45 (2005). https://doi.org/10.1007/s10329-004-0099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-004-0099-3