Abstract
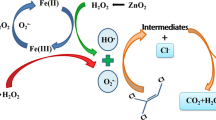
We developed an effective method for degradation of carbon tetrachloride (CT) in contaminated water. Zinc metal as a reducing agent for CT in aqueous solutions has been previously studied in some detail, but the rapid corrosion of zinc surface usually reduces its efficiency in removing CT. We assumed that citric acid could enhance the degradation of CT by zinc powder due to the elimination of a passivation layer of Zn(II) (hydr)oxides on the surface of zinc powder through chelating of organic ligands with Zn(II) produced from the reaction and keeping the exposure of active sites to targets. Here the influence of citric acid on the decomposing of CT by commercial micro-scale zinc powder was investigated in a pH range of 3.5–7.5 at 25°C in batch experiments. Reaction mixtures were analysed by gas chromatography/headspace analysis, and Cl− concentration was determined by turbidimetry. The results demonstrate that the degradation of CT by zinc metal alone is very weak, but the addition of citric acid can assist zinc powder to decompose CT more completely and rapidly at all pHs. Degradation of CT took place mainly in the first 10 min of reaction, coupled with 75–95% of CT removal. Maximum dechlorination percentage (82.4%) of CT was obtained at pH 5.5. In that case, chloroform and dichloromethane, as main intermediates, were found at low levels during the whole reaction, suggesting that CT may be sequentially and multiply degraded so quickly that methane is yielded before the intermediates can be desorbed and released to aqueous solution. When compared with the current methods of nano-scale zinc and bimetallic systems, the application of commercial micro-scale zinc particles assisted by organic ligands is of environmental significance since it allows decontamination of aqueous chlorinated organic compounds at low cost and with high efficiency.






Similar content being viewed by others
References
Agrawal A, Ferguson WJ, Gardner BO, Christ JA, Bandstra JZ, Tratnyek PG (2002) Effects of carbonate species on the kinetics of dechlorination of 1, 1, 1-trichloroethane by zero-valent iron. Environ Sci Technol 36:4326–4333
Boronina TN, Klabunde KJ, Sergeev GC (1995) Destruction of organohalides in water using metal particles: carbon tetrachloride/water reactions with magnesium, Tin, and Zinc. Environ Sci Technol 29:1511–1517
Boronina TN, Lagadic I, Sergeev GB, Klabunde KJ (1998) Activated and nonactivated forms of zinc powder: reactivity toward chlorocarbons in water and AFM studies of surface morphologies. Environ Sci Technol 32:2614–2622
Cheng SF, Wu SC (2000) The enhancement methods for the degradation of TCE by zero-valent metals. Chemosphere 41:1263–1270
Choi J, Choi K, Lee W (2009) Effect of transition metal and sulfide on the reductive dechlorination of carbon tetrachloride and 1, 1, 1-trichloroetgane by FeS. J Hazard Mater 162:115–1158
Clark CJ II, Rao PSC, Annable MD (2003) Degradation of perchloroethylene in cosolvent solution by zero-valent iron. J Hazard Mater 96:65–78
Feng J, Lim TT (2005) Pathways and kinetics of carbon tetrachloride and chloroform reductions by nano-scale Fe and Fe/Ni particles: comparison with commercial micro-scale Fe and Zn. Chemosphere 59:1267–1277
Fennelly JP, Roberts AL (1998) Reaction of 1, 1, 1-Trichloroethane with zero-valent metals and bimetallic reductants. Environ Sci Technol 32:1980–1988
Gillham RW, O’Hannesin SF (1994) Enhanced degradation of halogenated aliphatics by zero—valent iron. Gr Water 32:958–967
Kober R, Schlicker O, Ebert M, Dahmke A (2002) Degradation of chlorinated ethylenes by Fe (0): inhibition processes and mineral precipitation. Environ Geol 41:644–652
Li WF, Klabunde KJ (1998) Ultrafine zinc and nickel, palladium, silver coated zinc particles used for reductive dehalogenation of chlorinated ethylenes in aqueous solution. Croat Chem Acta 74:853–872
Mariaa LT, Lizaberth CB (2004) Effects of iron purity and groundwater characteristics on rates and products in the degradation of carbon tetrachloride by iron metal. Environ Sci Technol 38:1866–1876
Matheson LJ, Tratnyek PG (1994) Reductive dehalogenation of chlorinated methanes by iron metal. Environ Sci Technol 28:2045–2053
Roberts AL, Totten LA, Arnold WA, Boris DR, Campbell TJ (1996) Reductive elimination of chlorinated ethylenes by zero-valent metals. Environ Sci Technol 30:2654–2659
Song H, Carraway E (2006) Reduction of chlorinated ethanes by nano-sized zero-valent iron: kinetics, pathways and effects of reaction conditions. Environ Eng Sci 23:272–284
Sweeny KH (1979) American water works association research foundation. Denver, water reuse symposium 2:1487
Sweeny KH, Fischer JR (1972) Reductive degradation of halogenated pesticides. US Patent No. 3,640,821, 8 Feb 1972
Vogel TM, Criddle CS, McCarty PL (1987) ES critical reviews: transformations of halogenated aliphatic compounds. Environ Sci Technol 21:722–736
Wang A, Tang B, Hu X (2007) Determination of micro chloride ion in acidic copper electroplating solution by turbidimetric. Trace Elem Sci 14:45–47 (in Chinese)
Warren KD, Arnold RG, Bishop TL, Lindholm LC, Betterton EA (1995) Kinetics and mechanism of reductive dehalogenation of carbon tetrachloride using zero-valence metals. J Hazard Mater 41:217–227
William AA, William PB, Roberts AL (1999) Polychlorinated ethane reaction with zero-valent zinc: pathways and rate control. J Contam Hydrol 40:183–200
Zhang WX, Wang CB, Lien HL (1998) Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal Today 40:387–395
Acknowledgments
The authors would like to thank John R. Helms for editorial assistance during the revision of this manuscript. This study was supported by the Fundamental Research Funds for the Central Universities of China (Grant No. KYZ200918). Partial support by the National Science Foundation (EAR- 0843996 and CBET-0853950) is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, X., Yang, F., Lan, Y. et al. Rapid degradation of carbon tetrachloride by commercial micro-scale zinc powder assisted by citric acid. Environ Chem Lett 9, 431–438 (2011). https://doi.org/10.1007/s10311-010-0298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-010-0298-7